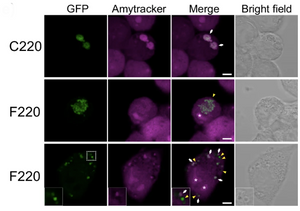
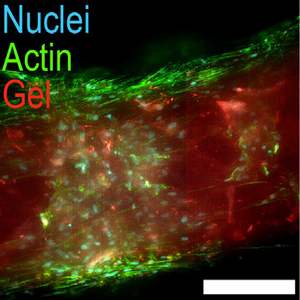
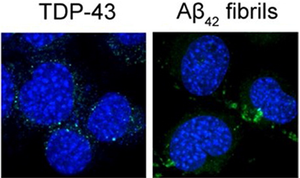
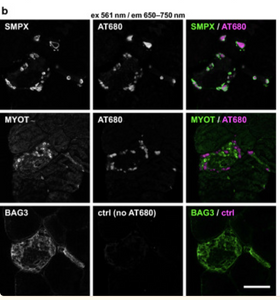
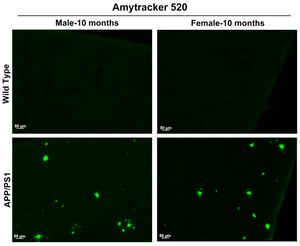
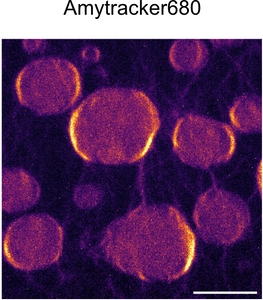
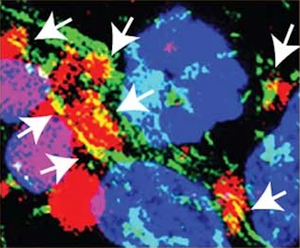
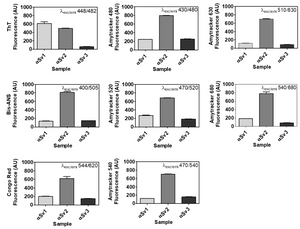
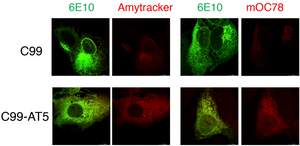
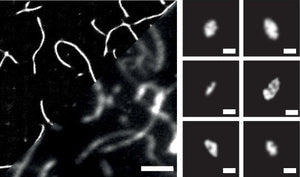
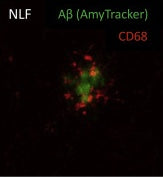
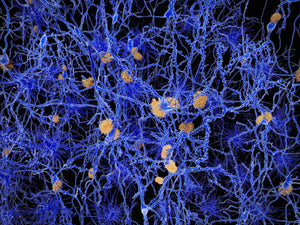
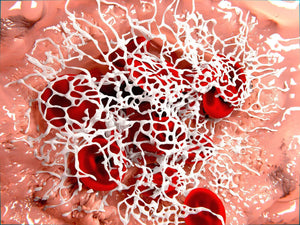
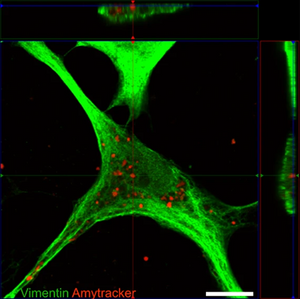
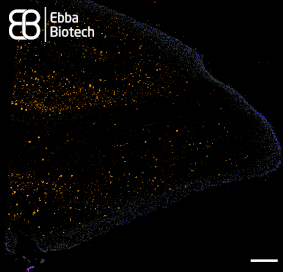
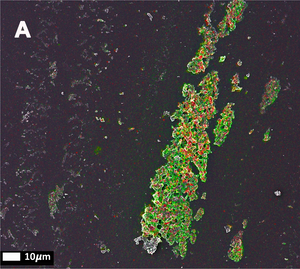
Amytracker are small fluorescent molecules for detection of protein aggregates.
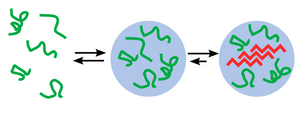
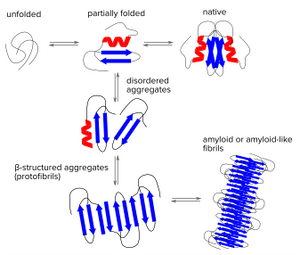
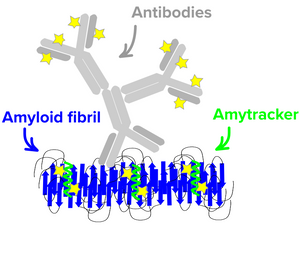
Five Amytracker variants are available. All Amytracker variants are designed to bind to the Congo red binding pocket on the amyloid fibril. A minimum of eight in-register parallel-β-strands are required for binding. The Amytracker variants differ in affinity and spectral properties. As Amytracker are structural markers, you can achieve reliable fluorescent labeling of amyloids derived from a variety of amyloidogenic proteins or peptides from different species.
Amytracker are suitable for detecting amyloids in fresh or fixed tissue sections and cells. It is possible to use them for fibrillation assays and for systemic injection in vivo. They are exceptionally photo- and thermostable and allow for easy handling in any application. Amytracker work in a wide range of salt and pH conditions. When the pH is altered during the experiment, pH controls should be included. Amytracker can be used with fluorescence plate readers, fluorescence microscopes and confocal laser scanning microscopes, fluorescence life time imaging, fluorescence cytometry, Total internal reflection fluorescence (TIRF) microscopy and Multiphoton microscopy.
Store your Amytracker product in the fridge and use the opened container within 12 months. Amytracker is for research use only and is not for resale.
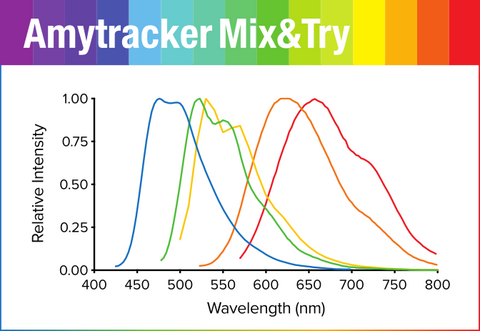
Amytracker Mix&Try is our recommended option for starting out with using Amytracker. It contains 10 µL of each variant. Testing the variants will allow you to determine which Amytracker is best suited for your experiments and available instruments.
All Amytracker variants label Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. The optotracers are exceptionally photostable and fluorogenic. The variants differ with regards to affinity, cellular uptake and excitation and emission wavelengths (see Table below). When bound to a target, Amytracker can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Contact us to learn more about Amytracker applications.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 480 | 420 nm | 480 nm | DAPI |
| Amytracker 520 | 460 nm | 520 nm | FITC, GFP |
| Amytracker 540 | 480 nm | 540 nm | FITC, GFP, YFP |
| Amytracker 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
| Amytracker 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |
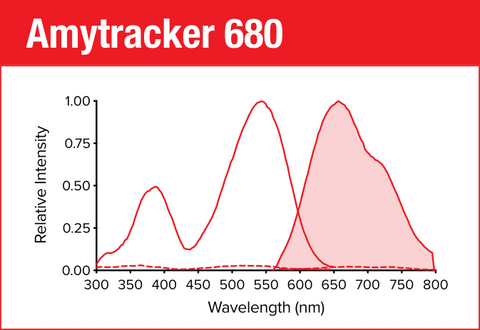
Amytracker 680 is our red optotracer for labeling protein aggregates with repetitive arrangement of β-sheets. It labels Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. Specifically, Amytracker 680 has been used to study amyloid formation during abnormal coagulation, Lewy body formation in a seeding based neuronal model, accumulation of misfolded proteins in the nucleolus and intracerebral formation of Aβ plaques using multiphoton microscopy. Contact us to learn more about Amytracker applications.
As all our optotracers, Amytracker 680 is exceptionally photostable and fluorogenic. When bound to a target, Amytracker 680 can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |
Amytracker 680 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Solid: 1 mg solid lyophilised in a sterile injection bottle. We recommend dilution to 4 mg/ml in physiological saline followed by intravenous injection with a total dose of 5 mg/KG.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect amyloids within minutes.
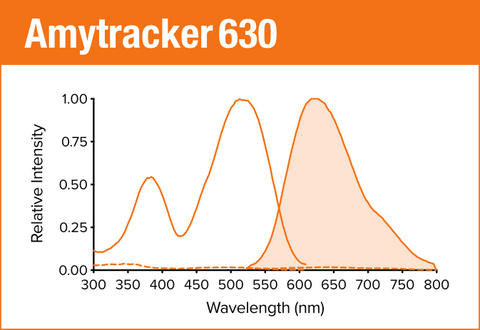
Amytracker 630 is our orange optotracer for labeling protein aggregates with repetitive arrangement of β-sheets. It labels Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. Contact us to learn more about Amytracker applications.
As all our optotracers, Amytracker 630 is exceptionally photostable and fluorogenic. When bound to a target, Amytracker 630 can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
Amytracker 630 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
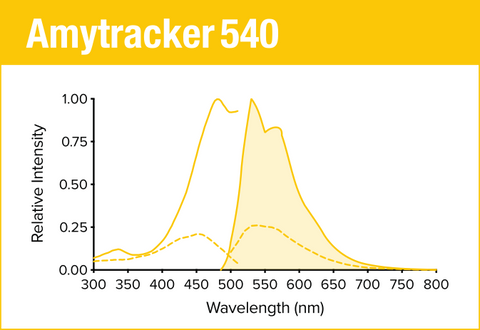
Amytracker 540 is our yellow optotracer for labeling protein aggregates with repetitive arrangement of β-sheets. It labels Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. Contact us to learn more about Amytracker applications.
As all our optotracers, Amytracker 540 is exceptionally photostable and fluorogenic. When bound to a target, Amytracker 540 can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 540 | 480 nm | 540 nm | FITC, GFP, YFP |
Amytracker 540 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
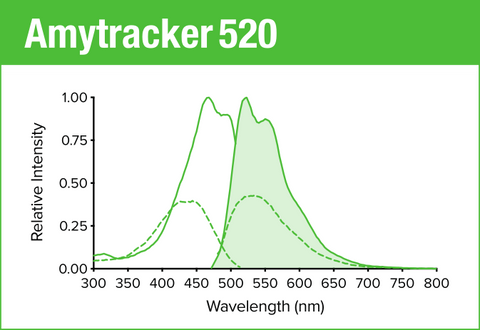
Amytracker 520 is our green optotracer for labeling protein aggregates with repetitive arrangement of β-sheets. It labels Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. Contact us to learn more about Amytracker applications.
As all our optotracers, Amytracker 520 is exceptionally photostable and fluorogenic. When bound to a target, Amytracker 520 can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 520 | 460 nm | 520 nm | FITC, GFP |
Amytracker 520 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
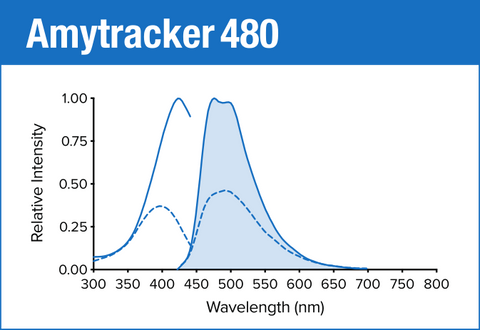
Amytracker 480 is our blue optotracer for labeling protein aggregates with repetitive arrangement of β-sheets. It labels Aβ plaques and neurofibrillary tangles in tissue sections with AD pathology and α-synuclein aggregates in tissue sections with PD pathology. Specifically, Amytracker 480 has been used to study amyloid formation during abnormal coagulation. Contact us to learn more about Amytracker applications.
As all our optotracers, Amytracker 480 is exceptionally photostable and fluorogenic. When bound to a target, Amytracker 480 can be imaged using epifluorescence, confocal and superresolution microscopy. Spectral information can be acquired using a fluorescence spectrophotometer. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Amytracker 480 | 420 nm | 480 nm | DAPI |
Amytracker 480 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
Labeling of protein aggregates in tissue sections or cells
Read more →
Amytracker for systemic injection
Read more →
Fibrillation assay
Read more →
Live-cell imaging
Read more →
Tracing Tau: A Step Closer to Understanding Neurodegeneration
Read more →
Finding the Invisible: How Amyloid Detection Is Improving
Read more →
Glycosylation guides a safer strain of α-synuclein
Read more →
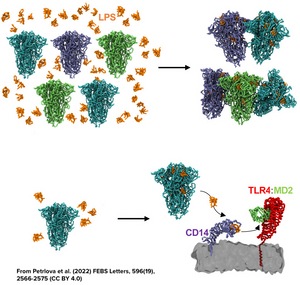
ApoE Aggregates and Innate Immunity: Insights into Endotoxin Sequestration
Read more →
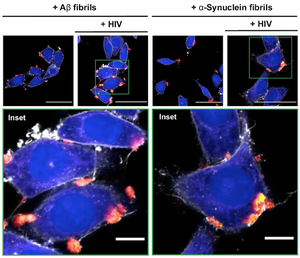
When Amyloids Meet Viruses: α-Synuclein Fibrils Enhance HIV-1 Infection
Read more →
Antibodies Against a Synthetic Aβ Trimer-Mimic to Map Amyloid Pathology
Read more →
Unraveling Tau Propagation: Amytracker Reveals Astrocytic Pathways
Read more →
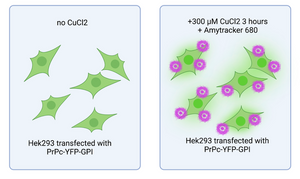
Copper’s role in prion protein misbehaviour
Read more →
How functional amyloids help regulate stress responses
Read more →
Amytracker aids in designing small molecules to inhibit aggregation of α-synuclein
Read more →
Oxidative stress and amyloid formation contributes to phenylketonuria disease
Read more →
The structural basis of TDP-43 aggregation
Read more →
Uncovering a new link to amyloid formation in neurodegenerative diseases
Read more →
Modular protein hydrogels for flexible applications
Read more →
Amyloid formation in ALS: Redefining the role of TDP-43 inclusions
Read more →
Amytracker confirms amyloid nature of inclusion bodies in muscle linked to gene mutations
Read more →
Estrogen might be women’s secret weapon!
Read more →
Transition to aggregation in hnRNPA1A condensates visualised using Amytracker
Read more →
Amytracker shows how α-Synuclein aggregates at the mitochondrial membrane
Read more →
Studying the relationship between morphology and inflammatory effect of ɑ-synuclein aggregates
Read more →
Faulty ribosome quality control triggers amyloid aggregation
Read more →
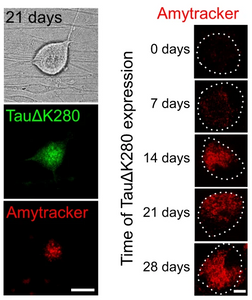
Amytracker labels amyloid forms of tau in a cell based model for tauopathies
Read more →
Amyloids trigger proinflammatory signalling Multiple Myeloma
Read more →
Amytracker: a tool to identify toxic dipeptide repeats
Read more →
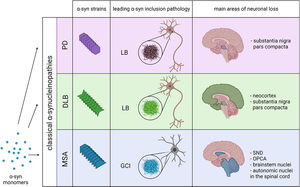
How mutations contribute to pathological diversity in synucleopathies
Read more →
Deciphering the complexity of intracellular Tau aggregates
Read more →
Linking aggregate size and toxicity using Amytracker
Read more →
Protein aggregation in wound healing
Read more →
Phase separated α-synuclein is more prone to aggregation
Read more →
FL-OPTIR for studying biophysical properties of amyloids in cells and tissues
Read more →
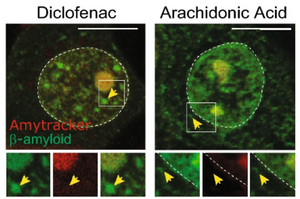
Spatial pattern of microglial activation in relation to amyloid plaques
Read more →
Amyloidogenicity of SARS-CoV-2 spike protein
Read more →
Lewy body formation in seeding based neuronal models
Read more →
Prolonged stress leads to accumulation of misfolded proteins in the nucleolus
Read more →
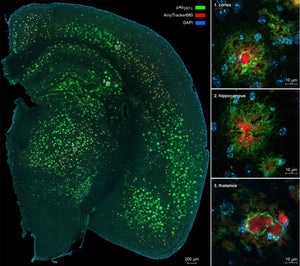
Amytracker can be used for intracerebral multiphoton microscopy
Read more →
The clue to detect multiple systems atrophy?
Read more →
Anomalous fibrin amyloid formation
Read more →
Amyloids in type-2 diabetes
Read more →
Advanced imaging
Read more →
Protein engineering for better PET radioligands
Read more →
Artifical amyloids
Read more →
Amytracker in action for Neuroprotection
Read more →
Studying the inflammation-aggregation connection using Amytracker
Read more →
Multi-Laser / Multi-Detector Imaging with Amytracker
Read more →
Optotracing using Amytracker
Read more →
Amytracker and Long Covid
Read more →
Amytracker and the age-pigment Lipofuscin
Read more →
Amytracker for the study of tau aggregates
Read more →
Amytracker for studying α-synuclein aggregates
Read more →
When proteins get out of shape
Read more →
Are amyloid structures in abnormal blood clots a risk factor for amyloidosis?
Read more →
Phase separation and protein aggregation
Read more →
Amytracker fluorescence spectra
Read more →
Amytracker compared to Congo Red
Read more →
Does Amytracker bind unspecifically?
Read more →
Fixation technique for Amytracker
Read more →
Amytracker for use in various tissues and species
Read more →
Amytracker binding
Read more →
Amytracker for detection of Amyloidosis
Read more →
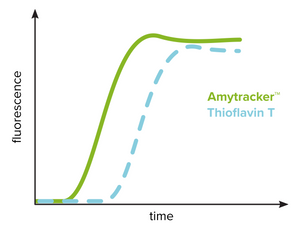
Amytracker compared to Thioflavin
Read more →
How should I dilute Amytracker?
Read more →
Amyloids - the dark matter of biology
Read more →
Amytracker for amyloid staining
Read more →
Amytracker to investigate amyloid formation
Read more →
Amytracker for super-resolution microscopy
Read more →
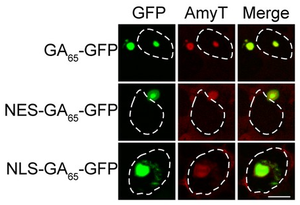
Amytracker for live cell Imaging
Read more →
In vivo amyloid staining and intravital imaging
Read more →
Testimonial - Leon Smyth
Read more →
Testimonial - Linh Tran
Read more →
Testimonial - Manuela Leri
Read more →
Testimonial - Azad Farzadfard
Read more →
Testimonial - reMynd
Read more →
Testimonial - Fabrizio Chiti
Read more →
Testimonial - Adam Kreutzer
Read more →
Testimonial - Keiza Jack
Read more →
Testimonial - Jaakko Sarparanta
Read more →
Testimonial - Megg Garcia
Read more →
Detection of functional amyloids in stress-treated mammalian cells
Read more →
Amytracker - A New Frontier in Imaging of Amyloid Structures in Tissues
Read more →
Amyloid fibril polymorphism in proteinopathies
Read more →
Consequences of coagulation in health and disease
Read more →
Optotracers - multifunctional fluorescent tracers
Read more →
Fluorescence microscopy techniques using Amytracker-like molecules
Read more →
Peter Nilsson develops multifunctional tools for diagnosis and therapy
Read more →
We named our Amytracker molecules after their peak emission wavelength when they are bound to their target. That means, when Amytracker is bound to a target, it will emit fluorescence at peak emission indicated by the number associated with its name.
To view the excitation and emission spectra, please select your Amytracker below :
2025
-
Puthia, M., Marzinek, J. K., Vesela, K., Larsson, A., Schmidtchen, A., Bond, P. J., & Petrlova, J. (2025). Apolipoprotein E3 and E4 isoforms exhibit differing effects in countering endotoxins. Journal of Biological Chemistry, 301(3), 108236. https://doi.org/10.1016/j.jbc.2025.108236
2024
-
Pinzi, L., Conze, C., Bisi, N., Torre, G. D., Soliman, A., Monteiro-Abreu, N., Trushina, N. I., Krusenbaum, A., Dolouei, M. K., Hellwig, A., Christodoulou, M. S., Passarella, D., Bakota, L., Rastelli, G., & Brandt, R. (2024). Quantitative live cell imaging of a tauopathy model enables the identification of a polypharmacological drug candidate that restores physiological microtubule interaction. Nature Communications, 15(1), 1679. https://doi.org/10.1038/s41467-024-45851-6
-
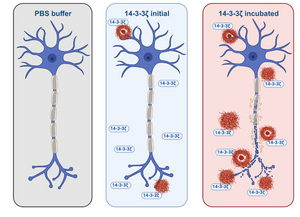
Šulskis, D., Žiaunys, M., Sakalauskas, A., Sniečkute, R., & Smirnovas, V. (2024). Formation of amyloid fibrils by the regulatory 14-3-3ζ protein. Open Biology, 14(1). https://doi.org/10.1098/rsob.230285
-
Dranseike, D., Ota, Y., Edwardson, T. G. W., Guzzi, E. A., Hori, M., Nakic, Z. R., Deshmukh, D. v., Levasseur, M. D., Mattli, K., Tringides, C. M., Zhou, J., Hilvert, D., Peters, C., & Tibbitt, M. W. (2024). Designed modular protein hydrogels for biofabrication. Acta Biomaterialia, 177, 107–117. https://doi.org/10.1016/J.ACTBIO.2024.02.019
-
Balana, A. T., Mahul-Mellier, A. L., Nguyen, B. A., Horvath, M., Javed, A., Hard, E. R., Jasiqi, Y., Singh, P., Afrin, S., Pedretti, R., Singh, V., Lee, V. M. Y., Luk, K. C., Saelices, L., Lashuel, H. A., & Pratt, M. R. (2024). O-GlcNAc forces an α-synuclein amyloid strain with notably diminished seeding and pathology. Nature Chemical Biology, 20(5), 646–655. https://doi.org/10.1038/s41589-024-01551-2
-
Kreutzer, A. G., Parrocha, C. M. T., Haerianardakani, S., Guaglianone, G., Nguyen, J. T., Diab, M. N., Yong, W., Perez-Rosendahl, M., Head, E., & Nowick, J. S. (2024). Antibodies Raised Against an Aβ Oligomer Mimic Recognize Pathological Features in Alzheimer’s Disease and Associated Amyloid-Disease Brain Tissue. ACS Central Science, 10(1), 104–121. https://doi.org/10.1021/acscentsci.3c00592
-
Raymundo, J. R., Zhang, H., Smaldone, G., Zhu, W., Daly, K. E., Glennon, B. J., Pecoraro, G., Salvatore, M., Devine, W. A., Lo, C. W., Vitagliano, L., & Marneros, A. G. (2024). KCTD1/KCTD15 complexes control ectodermal and neural crest cell functions, and their impairment causes aplasia cutis. The Journal of Clinical Investigation, 134(4). https://doi.org/10.1172/JCI174138
-
Morelli, C., Faltova, L., Capasso Palmiero, U., Makasewicz, K., Papp, M., Jacquat, R. P. B., Pinotsi, D., & Arosio, P. (2024). RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates. Nature Chemistry, 16(7), 1052–1061. https://doi.org/10.1038/s41557-024-01467-3
-
Kitamura, A., Fujimoto, A., Kawashima, R., Lyu, Y., Sasaki, K., Hamada, Y., Moriya, K., Kurata, A., Takahashi, K., Brielmann, R., Bott, L. C., Morimoto, R. I., & Kinjo, M. (2024). Hetero-oligomerization of TDP-43 carboxy-terminal fragments with cellular proteins contributes to proteotoxicity. Communications Biology, 7(1). https://doi.org/10.1038/s42003-024-06410-3
-
de Oliveira, D. H., Gowda, V., Sparrman, T., Gustafsson, L., Sanches Pires, R., Riekel, C., Barth, A., Lendel, C., & Hedhammar, M. (2024). Structural conversion of the spidroin C-terminal domain during assembly of spider silk fibers. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-49111-5
-
Sun, H., Yang, B., Li, Q., Zhu, X., Song, E., Liu, C., Song, Y., & Jiang, G. (2024). Polystyrene nanoparticles trigger aberrant condensation of TDP-43 and amyotrophic lateral sclerosis-like symptoms. Nature Nanotechnology. https://doi.org/10.1038/s41565-024-01683-5
-
Li, B., Suresh, P., Brelstaff, J., Kedia, S., Bryant, C. E., & Klenerman, D. (2024). The delayed kinetics of Myddosome formation explains why amyloid-beta aggregates trigger Toll-like receptor 4 less efficiently than lipopolysaccharide. eLife, 13, RP92350. https://doi.org/10.7554/eLife.92350
-
Bacioglu, M., Schweighauser, M., Gray, D., Lövestam, S., Katsinelos, T., Quaegebeur, A., van Swieten, J., Jaunmuktane, Z., Davies, S. W., Scheres, S. H. W., Goedert, M., Ghetti, B., & Spillantini, M. G. (2024). Cleaved TMEM106B forms amyloid aggregates in central and peripheral nervous systems. Acta Neuropathologica Communications, 12(1). https://doi.org/10.1186/s40478-024-01813-z
-
Eltom, K., Mothes, T., Libard, S., Ingelsson, M., & Erlandsson, A. (2024). Astrocytic accumulation of tau fibrils isolated from Alzheimer’s disease brains induces inflammation, cell-to-cell propagation and neuronal impairment. Acta Neuropathologica Communications, 12(1). https://doi.org/10.1186/s40478-024-01745-8
2023
-
Arad, E., Pedersen, K. B., Malka, O., Mambram Kunnath, S., Golan, N., Aibinder, P., Schiøtt, B., Rapaport, H., Landau, M., & Jelinek, R. (2023). Staphylococcus aureus functional amyloids catalyze degradation of β-lactam antibiotics. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-43624-1
-
Juliani do Amaral, M., Mohapatra, S., Ribeiro Passos, A., Sousa Lopes da Silva, T., Sampaio Carvalho, R., da Silva Almeida, M., Sá Pinheiro, A., Wegmann, S., & Cordeiro, Y. (2023). Copper drives prion protein phase separation and modulates aggregation. Science Advances, 9, eadi7347. https://doi.org/10.1126/sciadv.adi7347
-
Chandhok, S., Pereira, L., Momchilova, E. A., Marijan, D., Zapf, R., Lacroix, E., Kaur, A., Keymanesh, S., Krieger, C., & Audas, T. E. (2023). Stress-mediated aggregation of disease-associated proteins in amyloid bodies. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-41712-2
-
Chia, S., Faidon Brotzakis, Z., Horne, R. I., Possenti, A., Mannini, B., Cataldi, R., Nowinska, M., Staats, R., Linse, S., Knowles, T. P. J., Habchi, J., & Vendruscolo, M. (2023). Structure-Based Discovery of Small-Molecule Inhibitors of the Autocatalytic Proliferation of α-Synuclein Aggregates. Mol. Pharmaceutics, 20, 183–193. https://doi.org/10.1021/acs.molpharmaceut.2c00548
-
Frenkel, A., Zecharia, E., Gómez-Pérez, D., Sendersky, E., Yegorov, Y., Jacob, A., Benichou, J. I. C., Stierhof, Y. D., Parnasa, R., Golden, S. S., Kemen, E., & Schwarz, R. (2023). Cell specialization in cyanobacterial biofilm development revealed by expression of a cell-surface and extracellular matrix protein. Npj Biofilms and Microbiomes 2023 9:1, 9(1), 1–10. https://doi.org/10.1038/s41522-023-00376-6
-
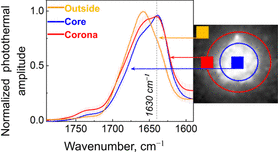
Gvazava, N., Konings, S. C., Cepeda-Prado, E., Skoryk, V., Umeano, C. H., Dong, J., Silva, I. A. N., Ottosson, D. R., Leigh, N. D., Wagner, D. E., & Klementieva, O. (2023). Label-Free High-Resolution Photothermal Optical Infrared Spectroscopy for Spatiotemporal Chemical Analysis in Fresh, Hydrated Living Tissues and Embryos. Journal of the American Chemical Society. https://doi.org/10.1021/jacs.3c08854
-
Petrlova, J., Hartman, E., Petruk, G., Lim, J. C. H., Adav, S. S., Kjellström, S., Puthia, M., & Schmidtchen, A. (2023). Selective protein aggregation confines and inhibits endotoxins in wounds: Linking host defense to amyloid formation. iScience, 26(10). https://doi.org/10.1016/j.isci.2023.107951
-
Kommaddi, R. P., Verma, A., Muniz-Terrera, G., Tiwari, V., Chithanathan, K., Diwakar, L., Gowaikar, R., Karunakaran, S., Malo, P. K., Graff-Radford, N. R., Day, G. S., Laske, C., Vöglein, J., Nübling, G., Ikeuchi, T., Kasuga, K., & Ravindranath, V. (2023). Sex difference in evolution of cognitive decline: studies on mouse model and the Dominantly Inherited Alzheimer Network cohort. Translational Psychiatry, 13(1), 1–12. https://doi.org/10.1038/s41398-023-02411-8
-
Ornithopoulou, E., Åstrand, C., Gustafsson, L., Crouzier, T., & Hedhammar, M. (2023). Self-Assembly of RGD-Functionalized Recombinant Spider Silk Protein into Microspheres in Physiological Buffer and in the Presence of Hyaluronic Acid. ACS Applied Bio Materials, 6(9), 3696–3705. https://doi.org/10.1021/acsabm.3c00373
-
Piroska, L., Fenyi, A., Thomas, S., Plamont, M.-A., Redeker, V., Melki, R., & Gueroui, Z. (2023). α-Synuclein liquid condensates fuel fibrillar α-synuclein growth. Science Advances, 9(33), eadg5663. https://doi.org/10.1126/sciadv.adg5663
-
Prater, C., Bai, Y., Konings, S. C., Martinsson, I., Swaminathan, V. S., Nordenfelt, P., Gouras, G., Borondics, F., & Klementieva, O. (2023). Fluorescently Guided Optical Photothermal Infrared Microspectroscopy for Protein-Specific Bioimaging at Subcellular Level. Journal of Medicinal Chemistry, 66(4), 2542–2549. https://doi.org/10.1021/acs.jmedchem.2c01359
2022
-
Cascella, R., Banchelli, M., Abolghasem Ghadami, S., Ami, D., Gagliani, M. C., Bigi, A., Staderini, T., Tampellini, D., Cortese, K., Cecchi, C., Natalello, A., Adibi, H., Matteini, P., & Chiti, F. (2022). An in situ and in vitro investigation of cytoplasmic TDP-43 inclusions reveals the absence of a clear amyloid signature. Annals of Medicine, 55(1), 72–88. https://doi.org/10.1080/07853890.2022.2148734
-
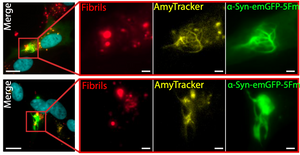
Choi, M. L., Chappard, A., Singh, B. P., Maclachlan, C., Abramov, A. Y., Horrocks, M. H., & Gandhi, S. (2022). Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity. Nature Neuroscience. https://doi.org/10.1038/s41593-022-01140-3
-
de Luca, C. M. G., Consonni, A., Cazzaniga, F. A., Bistaffa, E., Bufano, G., Quitarrini, G., Celauro, L., Legname, G., Eleopra, R., Baggi, F., Giaccone, G., & Moda, F. (2022). The alpha-synuclein RT-QuIC products generated by the olfactory mucosa of patients with parkinson’s disease and multiple system atrophy induce inflammatory responses in SH-SY5Y cells. Cells, 11(1). https://doi.org/10.3390/cells11010087
-
Wood, J. I., Wong, E., Cummings, D. M., Hardy, J., Correspondence, F. A. E., Joghee, R., Balbaa, A., Vitanova, K. S., Stringer, K. M., Vanshoiack, A., Phelan, S.-L. J., Launchbury, F., Desai, S., Tripathi, T., Rg Hanrieder, J., & Edwards, F. A. (2022). Plaque contact and unimpaired Trem2 is required for the microglial response to amyloid pathology. Cell Reports. https://doi.org/10.1016/j.celrep.2022.111686
-
Petrlova, J., Samsudin, F., Bond, P. J., & Schmidtchen, A. (2022). SARS-CoV-2 spike protein aggregation is triggered by bacterial lipopolysaccharide. FEBS Letters. https://doi.org/10.1002/1873-3468.14490
-
Morten, M. J., Sirvio, L., Rupawala, H., Hayes, E. M., Franco, A., Radulescu, C., Ying, L., Barnes, S. J., Muga, A., & Ye, Y. (2022). Quantitative super-resolution imaging of pathological aggregates reveals distinct toxicity profiles in different synucleinopathies. PNAS. https://doi.org/10.1073/pnas
-
Hochmair, J., Exner, C., Franck, M., Dominguez‐Baquero, A., Diez, L., Brognaro, H., Kraushar, M. L., Mielke, T., Radbruch, H., Kaniyappan, S., Falke, S., Mandelkow, E., Betzel, C., & Wegmann, S. (2022). Molecular crowding and RNA synergize to promote phase separation, microtubule interaction, and seeding of Tau condensates. The EMBO Journal, 41(11). https://doi.org/10.15252/EMBJ.2021108882
-
Kumar, S. T., Mahul-Mellier, A. L., Hegde, R. N., Rivière, G., Moons, R., de Opakua, A. I., Magalhães, P., Rostami, I., Donzelli, S., Sobott, F., Zweckstetter, M., & Lashuel, H. A. (2022). A NAC domain mutation (E83Q) unlocks the pathogenicity of human alpha-synuclein and recapitulates its pathological diversity. Science Advances, 8(17), 44. https://doi.org/10.1126/SCIADV.ABN0044
-
Lackie, R. E., de Miranda, A. S., Lim, M. P., Novikov, V., Madrer, N., Karunatilleke, N. C., Rutledge, B. S., Tullo, S., Brickenden, A., Maitland, M. E. R., Greenberg, D., Gallino, D., Luo, W., Attaran, A., Shlaifer, I., del Cid Pellitero, E., Schild-Poulter, C., Durcan, T. M., Fon, E. A., … Prado, M. A. M. (2022). Stress-inducible phosphoprotein 1 (HOP/STI1/STIP1) regulates the accumulation and toxicity of α-synuclein in vivo. Acta Neuropathologica. https://doi.org/10.1007/s00401-022-02491-8
2021
- Graziotto, M. E., Adair, L. D., Kaur, A., Vérité, P., Ball, S. R., Sunde, M., Jacquemin, D., & New, E. J. (2021). Versatile naphthalimide tetrazines for fluorogenic bioorthogonal labelling. RSC Chemical Biology, 2(5), 1491–1498. https://doi.org/10.1039/D1CB00128K
- Michno, W., Stringer, K. M., Enzlein, T., Passarelli, M. K., Escrig, S., Vitanova, K., Wood, J., Blennow, K., Zetterberg, H., Meibom, A., Hopf, C., Edwards, F. A., & Hanrieder, J. (2021). Following spatial Aβ aggregation dynamics in evolving Alzheimer’s disease pathology by imaging stable isotope labeling kinetics. Science Advances, 7(25), 4855–4871. https://doi.org/10.1126/SCIADV.ABG4855/
-
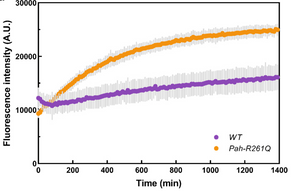
Aubi, O., Prestegård, K. S., Jung-KC, K., Shi, T. J. S., Ying, M., Grindheim, A. K., Scherer, T., Ulvik, A., McCann, A., Spriet, E., Thöny, B., & Martinez, A. (2021). The Pah-R261Q mouse reveals oxidative stress associated with amyloid-like hepatic aggregation of mutant phenylalanine hydroxylase. Nature Communications 2021 12:1, 12(1), 1–16. https://doi.org/10.1038/s41467-021-22107-1
-
Frey, B., AlOkda, A., Jackson, M. P., Riguet, N., Duce, J. A., & Lashuel, H. A. (2021). Monitoring alpha-synuclein oligomerization and aggregation using bimolecular fluorescence complementation assays: What you see is not always what you get. Journal of Neurochemistry, 157(4), 872–888. https://doi.org/10.1111/jnc.15147
-
Frottin, F., Pérez-Berlanga, M., Hartl, F. U., & Hipp, M. S. (2021). Multiple pathways of toxicity induced by C9orf72 dipeptide repeat aggregates and G4C2 RNA in a cellular model. ELife, 10. https://doi.org/10.7554/eLife.62718
-
Rimal, S., Li, Y., Vartak, R., Geng, J., Tantray, I., Li, S., Huh, S., Vogel, H., Glabe, C., Grinberg, L. T., Spina, S., Seeley, W. W., Guo, S., & Lu, B. (2021). Inefficient quality control of ribosome stalling during APP synthesis generates CAT-tailed species that precipitate hallmarks of Alzheimer’s disease. Acta Neuropathologica Communications, 9(1), 1–24. https://doi.org/10.1186/s40478-021-01268-6
-
Hofbauer, D., Mougiakakos, D., Mackensen, A., Ricagno, S., & Bruns, H. (2021). B2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity. https://doi.org/10.1016/j.immuni.2021.07.002
-
Johari, M., Sarparanta, J., Vihola, A., Jonson, P. H., Savarese, M., Jokela, M., Torella, A., Piluso, G., Said, E., Vella, N., Cauchi, M., Magot, A., Magri, F., Mauri, E., Kornblum, C., Reimann, J., Stojkovic, T., Romero, N. B., Luque, H., Huovinen, S., Lahermo, P., Donner, K., Comi, G. P., Nigro, V., Hackman, P., & Udd, B. (2021). Missense mutations in small muscle protein X-linked (SMPX) cause distal myopathy with protein inclusions. Acta Neuropathologica, 0123456789. https://doi.org/10.1007/s00401-021-02319-x
2020
-
Mahul-Mellier, A. L., Burtscher, J., Maharjan, N., Weerens, L., Croisier, M., Kuttler, F., Leleu, M., Knott, G. W., & Lashuel, H. A. (2020). The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America, 117(9), 4971–4982. https://doi.org/10.1073/pnas.1913904117
-
Ghosh, A., Mizuno, K., Tiwari, S. S., Proitsi, P., Gomez Perez-Nievas, B., Glennon, E., Martinez-Nunez, R. T., & Giese, K. P. (2020). Alzheimer’s disease-related dysregulation of mRNA translation causes key pathological features with ageing. Translational Psychiatry, 10(1), 1–18. https://doi.org/10.1038/s41398-020-00882-7
2019
-
Page, M. J., Thomson, G. J. A., Nunes, J. M., Engelbrecht, A. M., Nell, T. A., de Villiers, W. J. S., de Beer, M. C., Engelbrecht, L., Kell, D. B., & Pretorius, E. (2019). Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Scientific Reports, 9(1), 1–14. https://doi.org/10.1038/s41598-019-39056-x
-
Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., Kell, D. B., & Pretorius, E. (2019). Parkinson’s disease: A systemic inflammatory disease accompanied by bacterial inflammagens. Frontiers in Aging Neuroscience, 10(JUL), 1–17. https://doi.org/10.3389/fnagi.2019.00210
-
Frottin, F., Schueder, F., Tiwary, S., Gupta, R., Körner, R., Schlichthaerle, T., Cox, J., Jungmann, R., Hartl, F. U., & Hipp, M. S. (2019). The nucleolus functions as a phase-separated protein quality control compartment. Science, 365(6451), 342–347. https://doi.org/10.1126/science.aaw9157
2018
-
de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B., & Pretorius, E. (2018). Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson’s Disease, Alzheimer’s Disease and Type 2 Diabetes Mellitus. Scientific Reports, 8(1), 1–12. https://doi.org/10.1038/s41598-018-35009-y
-
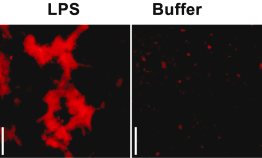
Pretorius, E., Page, M. J., Hendricks, L., Nkosi, N. B., Benson, S. R., & Kell, D. B. (2018). Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: Assessment with novel Amytracker TM stains. Journal of the Royal Society Interface, 15(139). https://doi.org/10.1098/rsif.2017.0941
2017
-
Sehlin, D., Fang, X. T., Meier, S. R., Jansson, M., & Syvänen, S. (2017). Pharmacokinetics, biodistribution and brain retention of a bispecific antibody-based PET radioligand for imaging of amyloid-β. Scientific Reports, 7(1), 1–9. https://doi.org/10.1038/s41598-017-17358-2
-
Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C., & Kell, D. B. (2017). Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovascular Diabetology, 16(1), 1–14. https://doi.org/10.1186/s12933-017-0624-5