HIV-associated neurocognitive disorders are observed in 30-50% of infected individuals, but they are poorly understood. A recent study published in Nature Communications uncovers a striking interaction between neurodegenerative disease-associated amyloids and HIV-1 infection.
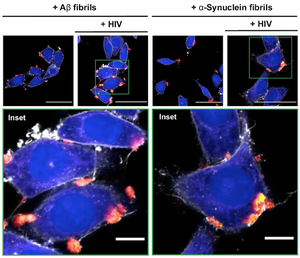
The group led by Frank Kirchhoff at Ulm Medical Center in Germany were able to visualize and confirm the presence of fibrillar α-synuclein and amyloid-beta (Aβ) in human cell lines, including T-cells and microglia. Using Amytracker 540 they revealed that these fibrils localize at the interface between virus and host cell, showing that they significantly enhance HIV-1 entry and replication in human cells. Amytracker made it possible to show how these fibrils act as physical bridges that facilitate viral attachment and infection. Interestingly, an HIV-1 envelope-derived amyloidogenic peptide was also shown to accelerate amyloid formation by α-synuclein and Aβ peptides. These insights suggest an interplay between viral infection and neurodegenerative disease-associated amyloids.
With its high sensitivity and spatial resolution, Amytracker proves to be a powerful tool for uncovering how amyloid structures can aid viral invasion. The findings pave the way for future research into anti-amyloid strategies as a potential means to mitigate amyloid-enhanced HIV-1 pathology.
Image: Using Amytracker 540 they stained amyloid fibrils formed from α-synuclein, Aβ. These stained fibrils (red) were then incubated with labeled HIV-1 particles (yellow) and target cells stained with cytoskeletal dyes (blue and white). Confocal microscopy images revealed that the amyloid fibrils bind HIV-1 particles and facilitate their attachment to the surface of target cells. Image adapted from Figure 5A by Olari, L.R., Liu, S., Arnold, F. et al. (2025) Nat Commun 16, 813 (CC BY 4.0).
