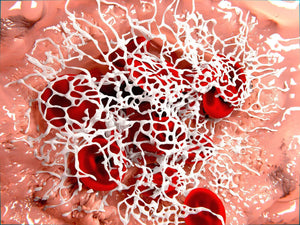
Lipopolysaccharides (LPS) from the Gram-negative cell envelope can be shed from dormant bacteria or from continual bacteria entry into the blood and serve to contribute to the chronic inflammation. The presence of highly substoichiometric amounts of LPS from Gram-negative bacteria caused fibrinogen clotting to lead to the formation of an amyloid form of fibrin. The teams around Prof. Douglas B. Kell from the University of Manchester and Prof. Etheresia Pretorius from the Stellenbosh University have shown that the broadly equivalent lipoteichoic acids (LTAs) from two species of Gram-positive bacteria have similarly (if not more) potent effects than LPS. Specifically they have showed that LPS, iron, and the 2 LTAs cause amyloid formation of plasma proteins, and in particular of fibrin(ogen) as blood is clotted. They confirmed amyloidogenesis by using Amytracker 480 and Amytracker 680 to identify amyloid protein deposits.
