The 14-3-3 proteins regulate various cellular functions, including enzymatic activity and protein stability. The 14-3-3ζ isoform has been linked to neurodegenerative diseases due to its interaction with proteins like tau and α-synuclein, which form amyloid fibrils in Alzheimer’s and Parkinson’s. However, its direct role in amyloid plaque formation remains unclear.
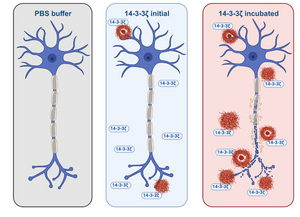
A group of researchers from the Institute of Biotechnology in Vilnius aimed to determine if 14-3-3ζ can form amyloid fibrils. Using bioinformatic tools, they identified several aggregation-prone regions. To test amyloid formation under physiological conditions, they incubated 14-3-3ζ and monitored the process using amyloid-specific dyes: Thioflavin T, Congo Red, and Amytracker 630. Amytracker 630, which binds to amyloid fibrils, showed a significant increase in fluorescence, indicating the aggregation of 14-3-3ζ into amyloid-like structures. Additional methods, including FTIR, circular dichroism, and AFM, confirmed the structural transition from a helical structure to β-sheet-rich amyloid fibrils.
The study demonstrated that 14-3-3ζ forms amyloid fibrils, suggesting it may contribute to amyloid pathology in neurodegenerative diseases. This finding opens up new avenues for exploring the role of 14-3-3 proteins in neuropathies and highlights potential therapeutic strategies targeting this protein. The researchers also found that 14-3-3ζ amyloid formation reduced cell viability by 65% in neuroblastoma assays, indicating its neurotoxic effects.
Image: Amytracker 630, a dye that binds to protein aggregates, showed a slight increase in fluorescence upon the initial addition of 14-3-3ζ protein. However, a significant rise in fluorescence was observed during the incubation period, suggesting that 14-3-3ζ forms amyloid fibrils over time. Image created with BioRender.
