Infrared (IR) spectroscopy is an invaluable tool to study the biophysical properties of amyloid aggregates. Although historically used as a tool for composition analysis of chemical compounds as it measures the vibrational energy of chemical bonds, IR spectroscopy provides measures to analyse the size and rigidity of amyloid fibrils. Coupling of IR spectroscopy to an optical microscope (OPTIR) is a new method that allows users to obtain chemical information of biological macromolecules from cells and tissues at submicron resolution. Using OPTIR for investigating the biophysical properties of amyloid fibrils in cells and tissues has been challenging since amyloid fibrils cannot be easily identified by conventional light microscopy. Therefore, Oxana Klementieva and her research group at Lund University in Sweden combined epifluorescence imaging and OPTIR in a single instrument. Their “Fluorescence-Located” OPTIR or FL-OPTIR allows precise targeting using epifluorescence signals to locate amyloid-rich regions for OPTIR analysis.
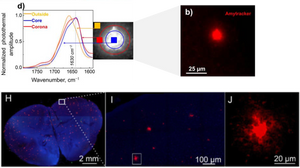
In a study published in Journal of Medicinal Chemistry, Prater et al. used fixed sections of APP/PS1 transgenic mouse brain and labelled amyloids using Amytracker 520. Guiding OPTIR measurements via the fluorescent signal from Amytracker 520, detailed biophysical analysis of amyloids in the core, corona, and outside of the plaque could be performed indicating a high degree of heterogeneity in the distribution of β-sheet structures within the amyloid plaque. Therefore, the FL-OPTIR method with the optotracer Amytracker might be valuable in studying amyloids in cells or tissue.
In a subsequent study, Gvazava et al. demonstrated that OPTIR can be applied to fresh, hydrated, and unprocessed mouse tissue biopsies, including brain tissue. Following OPTIR spectroscopy, Amytracker 520 was used to confirm the presence of amyloid plaques in freshly prepared brain slices from APP/PS1 transgenic mice, validating that OPTIR imaging can detect amyloid structures directly in living tissue. This study highlights a need of assessing protein structure in fresh, unprocessed tissue, as conventional fixation and dehydration were shown to alter amyloid β-sheet organization.
Altogether, these studies demonstrate the applicability of (FL-)OPTIR for spatiotemporal chemical analysis of amyloid plaques in both fixed and living tissue, representing a breakthrough in IR spectroscopic imaging of complex biological systems.
Image: Upper panels: FL-OPTIR spectra recorded from the outside, core, and plaque corona as indicated by the markers of the corresponding colour on the inset representing an amyloid plaque stained with Amytracker 520 (red) in fixed tissue. Adapted from Figure 2B and D, Prater et al. 2023, (CC BY 4.0). Lower panels: Amytracker 520-labeled amyloid plaques (red) in fresh, unprocessed mouse brain tissue (blue autofluorescence) following OPTIR measurements. Adapted from Figure 2H-J, Gvazava et al. 2023 (CC BY 4.0).
Read More:
- Prater, C. et al. (2023) Fluorescently Guided Optical Photothermal Infrared Microspectroscopy for Protein-Specific Bioimaging at Subcellular Level. Journal of Medicinal Chemistry, 66(4), 2542−2549
- Gvazava, N. et al. (2023) Label-Free High-Resolution Photothermal Optical Infrared Spectroscopy for Spatiotemporal Chemical Analysis in Fresh, Hydrated Living Tissues and Embryos. Journal of the American Chemical Society, 145 (45), 24796-24808
