Around 30% of the people infected with the SARS-CoV-2 virus report persistent symptoms for a long time after the acute infection ends. While the development of this long-term manifestation after COVID, referred to as Long COVID, has been framed as mysterious, it is actually a well described outcome of many viral or bacterial infections. In the case of Long COVID, the chronic symptoms include shortness of breath, fatigue, chest pain, headaches, “brain fog”, and a procoagulant state.
However common, it is not clear how the virus mediates these prolonged effects. Several hypotheses have been proposed to try and explain the phenomenon: Some experts propose that SARS-CoV-2 is able to reach and infect the cells of the central nervous system and infect neurons, thus causing neuroinflammation. Others believe that the virus might remain active in certain tissues and not be cleared completely from the body. Finally, it has been suggested that the virus affects the microbiome and the endothelium, allowing bacteria to enter the bloodstream.
This last hypothesis is particularly interesting in light of the work of Prof. Resia Pretorius from the University of Stellenbosch in South Africa. In her early career, she worked on the “bacteria in blood hypothesis” suggesting that bacteria from the gut and the oral mucosa might exist in the blood in a dormant state. During intense periods of stress, these bacteria might exit dormancy and shed inflammagens, such as LPS or other Endotoxins, causing generalized inflammation and abnormal clotting involving the formation of fibrin amyloids, which can be identified using Amytracker. As abnormal clotting has been linked to several inflammatory diseases like Diabetes and Parkinson's Disease by Pretorius et al (Read more here), it provides a connection between inflammation and protein aggregation, which can be identified using Amytracker. When the COVID-19 pandemic hit, Prof. Pretorius turned to the blood of COVID patients, and found that the infection caused the formation of the same kind of abnormal blood clots that she described before. These amyloid clots can reach 200 μm in size, meaning that they can effectively block microcapillaries and thus reduce the oxygen supply to tissues. A reduction in oxygen availability could, by itself, explain many of the symptoms of both acute and long COVID, providing a way in which a predominantly respiratory disease could lead to damage to organs like the kidney, the muscles, and the central nervous system.
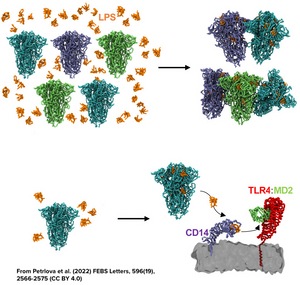
In addition, the work from Nyström & Hammarström from Linköping University in Sweden has shown how certain regions of the spike protein produced by SARS-CoV-2 have amyloidogenic properties, while Petrlova et al. from Prof. Schmittchen’s group at the University of Lund in Sweden recently demonstrated that LPS can trigger the aggregation of the spike protein as a whole in structures that are labeled using Amytracker (See feature image and read more here)
Taken together, these findings indicate that Long COVID might be an amyloid disease or amyloid-related disease caused by hyperinflammation as well as the presence of viral components and bacterial inflammagens in the blood. Although these findings are not conclusive and a diagnostic assay for Long COVID is still to be developed, we might consider abnormal clotting and amyloid formation as one of the puzzle pieces towards solving the Long COVID mystery.
Read More:
- Pretorius, E. et al. (2021) Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovascular Diabetology, 20(1), 172
- Nyström, S. & Hammarström, P. (2022) Amyloidogenesis of SARS-CoV-2 Spike Protein. Journal of the American Chemical Society, 144(20), 8945–8950
- Kell, D.B. et al. (2022) A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochemical Journal, 479(4), 537–559
- Petrlova, J. et al. (2022) SARS-CoV-2 spike protein aggregation is triggered by bacterial lipopolysaccharide. FEBS Letters, 596(19), 2566–2575
