Apolipoprotein E (ApoE) is widely recognized as a key genetic risk factor for Alzheimer’s disease.It is also implicated in other protein aggregation disorders such as Parkinson’s disease and Lewy Body dementia. Humans express three main alleles of the APOE gene, each associated with different levels of disease risk - APOE4, for instance, increases the risk of Alzheimer’s diseases by up to 15-fold in homozygotes, whereas APOE2 has a protective effect. Although the primary physiological function of ApoE is to mediate lipid transport and cholesterol metabolism throughout the body, it is also prone to aggregation and suggested to seed amyloid-β plaques.
In a recent study published in the Journal of Biological Chemistry, the group led by Jitka Petrlova, described a novel function for ApoE aggregation: serving as a defense mechanism against bacterial endotoxins. Using a range of biophysical techniques, the authors show how ApoE can bind and sequester lipopolysaccharides (LPS) from Gram-negative bacteria by aggregating around them. These LPS-ApoE aggregates facilitate macrophage uptake and appear to reduce local inflammation, potentially aiding disease outcomes.
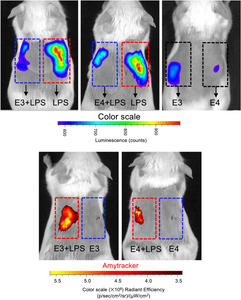
A highlight was the use of Amytracker 680 to track the formation of ApoE aggregates induced by LPS, both in vitro and in vivo. Amytracker 680 not only validated the structural changes observed through other methods but also provided a clear visual representation of how the different ApoE isoforms behave when challenged with LPS.
Overall, this work offers critical insights into how ApoE influences local inflammatory responses. Insights that could have significant impact on how we understand and potentially treat conditions where inflammation plays a key role, including sepsis and even some neurodegenerative diseases.
Overall, this work offers critical insights into the role of ApoE in modulating local inflammatory responses, which could have significant implications for how we understand and treat conditions where inflammation plays a key role, including sepsis and various neurodegenerative diseases.
Image: In vivo imaging by IVIS system of an inflammation reporter mouse. This mouse strain produces luminescence proportional to the activation of NF-κB, and shows how the inflammation signalling spreads after subcutaneous injection of LPS with or without different ApoE isoforms (Top). At the bottom, the same imaging system was used to detect ApoE aggregates in vivo using Amytracker 680 (red). Image adapted from Figure 10 by Puthia, M., Marzinek, J.K., Vesela, K. et al (2025) J Biol Chem. Volume 301, Issue 3, 108236 (CC BY 4.0).
