EbbaBiolight
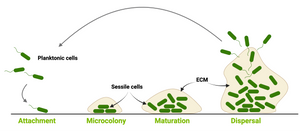
EbbaBiolight are optotracers for detection of bacteria & components of bacterial biofilm.
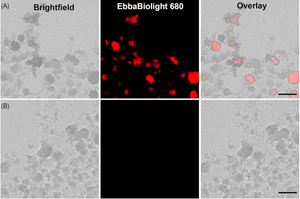
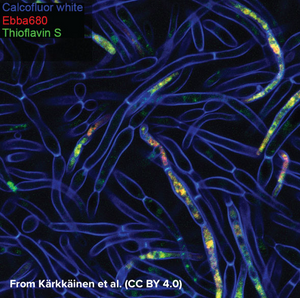
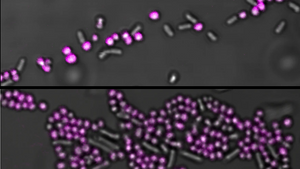
EbbaBiolight is available in five variants which label extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Specifically, EbbaBiolight variants have been used as follows: EbbaBiolight 480 labels extracellular matrix produced by P. aeruginosa but not C. albicans in a mircofluidic device during mono- and co-culture. EbbaBiolight 630 and EbbaBiolight 680 label peptidoglycan and lipoteichoic acids in the cell wall of certain gram positives like Staphylococci and E. faecalis. EbbaBiolight 680 labels curli in the extracellular matrix produced by Salmonella as well as β-glucans and chitins in yeast- and hypal forms of C. albicans. It has been used to track pellicle formation in B. cenocepacia and label extracellular matrix components in Pseudomonas and E. coli.
All EbbaBiolight variants are exceptionally photostable and fluorogenic. When bound to a target, the optotracers can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. All EbbaBiolight variants are intended for use in live-cultures. Fixation can lead to unspecific labelling of bacterial cells, regardless of them gram status. If fixation needs to be performed, we recommend to apply fixation after labelling.
EbbaBiolight variant work in a wide range of salt and pH conditions. When the pH is altered during the experiment, pH controls should be included. EbbaBiolight can be used with fluorescence plate readers, fluorescence microscopes and confocal laser scanning microscopes, fluorescence life time imaging, fluorescence cytometry, Total internal reflection fluorescence (TIRF) microscopy and Multiphoton microscopy.
Store your EbbaBiolight product in the fridge and use the opened container within 12 months. EbbaBiolight is for research use only and is not for resale.

EbbaBiolight Mix&Try is our recommended option for starting out with using EbbaBiolight. It contains 10 µL of each variant. Testing each variant in the will allow you to determine which one of our optotracers is best suited for your experiment.
All EbbaBiolight variants label extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Specifically, the optotracers have been used as follows: EbbaBiolight 480 labeling extracellular matrix produced by P. aeruginosa but not C. albicans in a mircofluidic device during mono- and co-culture. EbbaBiolight 630 labeling peptidoglycan and lipoteichoic acids in the cell wall of certain gram positives like Staphylococci and E. faecalis. EbbaBiolight 680 labeling curli in the extracellular matrix produced by Salmonella as well as β-glucans, chitin and intracellular amyloid aggregates in yeast- and hypal forms of C. albicans. Furthermore, it has been used to track pellicle biofilm formation in B. cenocepacia. Contact us to learn more about EbbaBiolight applications.
All EbbaBiolight variants are exceptionally photostable and fluorogenic. When bound to a target, the optotracers can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the following table.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 480 | 420 nm | 480 nm | DAPI |
| EbbaBiolight 520 | 460 nm | 520 nm | FITC, GFP |
| EbbaBiolight 540 | 480 nm | 540 nm | FITC, GFP, YFP |
| EbbaBiolight 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
| EbbaBiolight 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |

EbbaBiolight 680 is our red optotracer for labeling extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Specifically, EbbaBiolight 680 labels curli in the extracellular matrix produced by Salmonella as well as β-glucans and chitins in yeast- and hyphal forms of C. albicans. Furthermore, it has been used to track pellicle biofilm formation by B. cenocepacia. Contact us to learn more about EbbaBiolight applications.
As all our optotracers, EbbaBiolight 680 is exceptionally photostable and fluorogenic. When bound to a target, EbbaBiolight 680 can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the table below.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |
EbbaBiolight 680 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Solid: 1 mg solid lyophilised in a sterile injection bottle. We recommend dilution to 4 mg/ml in physiological saline followed by intravenous injection with a total dose of 5 mg/KG.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.

EbbaBiolight 630 is our orange optotracer for labeling extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Specifically, EbbaBiolight 630 labels peptidoglycan and lipoteichoic acids in the cell wall of certain gram positives like Staphylococci and E. faecalis. Contact us to learn more about EbbaBiolight applications.
As all our optotracers, EbbaBiolight 630 is exceptionally photostable and fluorogenic. When bound to a target, EbbaBiolight 630 can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the table below.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
EbbaBiolight 630 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Solid: 1 mg solid lyophilised in a sterile injection bottle. We recommend dilution to 4 mg/ml in physiological saline followed by intravenous injection with a total dose of 5 mg/KG.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.

EbbaBiolight 540 is our yellow optotracer for labeling extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Contact us to learn more about EbbaBiolight applications.
As all our optotracers, EbbaBiolight 540 is exceptionally photostable and fluorogenic. When bound to a target, EbbaBiolight 540 can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the table below.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 540 | 480 nm | 540 nm | FITC, GFP, YFP |
EbbaBiolight 540 is available in three different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.

EbbaBiolight 520 is our green optotracer for labeling extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Contact us to learn more about EbbaBiolight applications.
As all our optotracers, EbbaBiolight 520 is exceptionally photostable and fluorogenic. When bound to a target, EbbaBiolight 520 can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the table below.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 520 | 460 nm | 520 nm | FITC, GFP |
EbbaBiolight 520 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Solid: 1 mg solid lyophilised in a sterile injection bottle. We recommend dilution to 4 mg/ml in physiological saline followed by intravenous injection with a total dose of 5 mg/KG.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.

EbbaBiolight 480 is our blue optotracer for labeling extracellular and intracellular bacterial amyloids as well as certain glucans produced as part of the extracellular matrix. Specifically, EbbaBiolight 480 has been used to label extracellular matrix produced by P. aeruginosa but not C. albicans in a mircofluidic device during mono- and co-culture. Contact us to learn more about EbbaBiolight applications.
As all our optotracers, EbbaBiolight 480 is exceptionally photostable and fluorogenic. When bound to a target, EbbaBiolight 680 can be imaged using fluorescence microscopy and spectral information can be acquired using a fluorescence spectrophotometer. As spectral information can give hints of the nature of the target, we recommend to acquire excitation and emission spectra whenever possible. Use recommended filter sets as well as excitation- and emission wavelengths according to the table below.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| EbbaBiolight 480 | 420 nm | 480 nm | DAPI |
EbbaBiolight 480 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use. For use in live-cells, sometimes 1:500 is necessary due to uptake limitations.
- Solid: 1 mg solid lyophilised in a sterile injection bottle. We recommend dilution to 4 mg/ml in physiological saline followed by intravenous injection with a total dose of 5 mg/KG.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in tissue sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.
Monitoring curli production in liquid culture using EbbaBiolight
Read more →
Monitoring curli in bacterial biofilms forming on semi-solid agar
Read more →
Labeling of surface biofilm using EbbaBiolight
Read more →
How biofilm structural properties regulate nanoparticle distribution
Read more →
Bacterial Amyloids' Role in Autoimmune Inflammation
Read more →
The role of polyP in biofilm formation of hypervirulent Klebsiella pneumoniae
Read more →
Real-time insights into curli and cellulose dynamics in UPEC
Read more →
Use of EbbaBiolight in the development of a new root canal sterilisation tool
Read more →
How EbbaBiolight helps researchers to develop biofilm models
Read more →
Biofilm formation of UPEC in a biomimetic device occurs at a late stage of colonisation
Read more →
Antibiotic Susceptibility of Salmonella using EbbaBiolight supplemented agar
Read more →
Uncovering bacteria & funghi interactions
Read more →
Tracking of biofilm formation in Burkholderia
Read more →
Tracking fungal biofilm formation
Read more →
Watching bacterial cities grow
Read more →
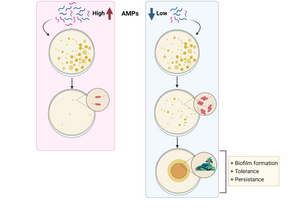
Are antimicrobial peptides ending the Antibiotic Crisis?
Read more →
EbbaBiolight-like molecule for detection and quantification of bacteria
Read more →
EbbaBiolight-like molecule detects biomarker for recurrent infection
Read more →
EbbaBiolight-like molecules reveals Salmonella biofilm secrets
Read more →
EbbaBiolight NBIC Interview
Optotracing with EbbaBiolight
Read more →
Antimicrobial peptides for targeting bacterial resistance
Read more →
EbbaBiolight fluorescence spectra
Read more →
Testimonial - Andrea Sass
Read more →
Testimonial - Cameron Croft
Read more →
Testimonial - Herve Straub
Read more →
What is the role of biofilms in urinary tract infections?
Read more →
Opto-electronically active Materials for Infection Detection and Control
Read more →
Chemical sensors enable scientists to watch bacterial cities grow
Read more →
Discovering an Antibiofilm Therapy for Urinary Tract Infections
Read more →
Optotracers - multifunctional fluorescent tracers
Read more →
Optotracing for detection & quantification of Staphylococci
Read more →
Agneta Richter-Dahlfors explains why biofilm is so important
Read more →
Cellulose in urine
Read more →
EbbaBiolight-like Molecule used for detection of cellulose in urine
Read more →
We named our EbbaBiolight molecules after their peak emission wavelength when they are bound to their target. That means, when EbbaBiolight is bound to a target, it will emit fluorescence at peak emission indicated by the number associated with its name.
To view the excitation and emission spectra, please select your EbbaBiolight below :
2025
-
Laudazzi, M., Schifano, E., Sivori, F., Altieri, L., Uccelletti, D., di Domenico, E. G., Colonna, B., Pasqua, M., & Prosseda, G. (2025). The AcrAB efflux pump contributes to the virulence of Enteroaggregative E. coli by influencing the aggregative behavior. Frontiers in Cellular and Infection Microbiology, 15, 1633585. https://doi.org/10.3389/fcimb.2025.1633585
-
Lavrikova, A., Janda, M., Bujdáková, H., & Hensel, K. (2025). Eradication of single- and mixed-species biofilms of P. aeruginosa and S. aureus by pulsed streamer corona discharge cold atmospheric plasma. Science of The Total Environment, 959, 178184. https://doi.org/10.1016/J.SCITOTENV.2024.178184
-
Guagliano, G., Peluso, E., Butnarasu, C. S., Restivo, E., Sardelli, L., Frasca, E., Petrini, P., Tirelli, N., Sganga, S., Visai, L., & Visentin, S. (2025). Mucosomes as next-generation drug carriers for treating mucus-resident bacterial infections and biofilms. Scientific Reports, 2025 15:1, 15(1), 27071-. https://doi.org/10.1038/s41598-025-10496-y
-
Peng, Z., Piaggio, A. L., Giglio, G. L., Ortega, S. T., van Loosdrecht, M. C. M., & de Kreuk, M. K. (2025). Interaction of non-biodegradable particles and granular sludge in Nereda®—— from nanoparticles to microparticles. Water Research, 281, 123698. https://doi.org/10.1016/J.WATRES.2025.123698
-
Silva, P. D. C., Hill, D., & Harrison, F. (2025). Optimizing synthetic cystic fibrosis sputum media for growth of non-typeable Haemophilus influenzae. Access Microbiology, 7(6), 000979.v3. https://doi.org/10.1099/acmi.0.000979.v3
-
Agresti, L., Boonstra, E. C., Jutte, P. C., van der Mei, H. C., & Sjollema, J. (2025). The applicability of fluorescent optotracers for in vitro and in vivo Staphylococcus aureus detection and quantification. Scientific Reports, 15:1, 15(1), 34503-. https://doi.org/10.1038/s41598-025-17029-7
-
Romero, A. I., Surkov, S., Wirsén, P., Brookes, G., Bergström, L., Tejbrant, J., Dhamo, E., Wilks, S., Bryant, C., & Andersson, J. (2025). LubriShieldTM—A permanent urinary catheter coating that prevents uropathogen biofilm formation in vitro independent of host protein conditioning. Scientific Reports, 15(1), 38221-. https://doi.org/10.1371/JOURNAL.PONE.0328167
-
Zhang, T., Bär, J., Risberg, L., Gómez Mejia, A., Hammar, H., Löffler, S., Otzen, D. E., Andreasen, M., Meyer, R. L., Melican, K., Zinkernagel, A. S., & Richter-Dahlfors, A. (2025). Dynamic visualization of extracellular matrix components in S. aureus colony biofilms reveals functional amyloids leading to the formation of cap-like structures. Biofilm, 10, 100318. https://doi.org/10.1016/J.BIOFLM.2025.100318
2024
-
Grando, K., Bessho, S., Harrell, K., Kyrylchuk, K., Pantoja, A. M., Olubajo, S., Albicoro, F. J., Klein-Szanto, A., & Tükel, Ç. (2024). Bacterial amyloid curli activates the host unfolded protein response via IRE1α in the presence of HLA-B27. Gut Microbes, 16(1), 2392877. https://doi.org/10.1080/19490976.2024.2392877
-
Rojas, D., Marcoleta, A. E., Gálvez-Silva, M., Varas, M. A., Díaz, M., Hernández, M., Vargas, C., Nourdin-Galindo, G., Koch, E., Saldivia, P., Vielma, J., Gan, Y.-H., Chen, Y., Guiliani, N., & Chávez, F. P. (2024). Inorganic Polyphosphate Affects Biofilm Assembly, Capsule Formation, and Virulence of Hypervirulent ST23 Klebsiella pneumoniae. ACS Infectious Diseases, 10(2), 606–623. https://doi.org/10.1021/acsinfecdis.3c00509
-
Zhang, T., Ray, S., Melican, K., & Richter-Dahlfors, A. (2024). The maturation of native uropathogenic Escherichia coli biofilms seen through a non-interventional lens. Biofilm, 8, 100212. https://doi.org/https://doi.org/10.1016/j.bioflm.2024.100212
-
Ray, S., Löffler, S., & Richter-Dahlfors, A. (2024). High-Resolution Large-Area Image Analysis Deciphers the Distribution of Salmonella Cells and ECM Components in Biofilms Formed on Charged PEDOT:PSS Surfaces. Advanced Science. https://doi.org/10.1002/advs.202307322
2023
-
Antypas, H., Zhang, T., Choong, F. X., Melican, K., & Richter-Dahlfors, A. (2023). Dynamic single cell analysis in a proximal-tubule-on-chip reveals heterogeneous epithelial colonization strategies of uropathogenic Escherichia coli under shear stress. FEMS Microbes, 4, 1–12. https://doi.org/10.1093/femsmc/xtad007
-
Richter-Dahlfors, A., Kärkkäinen, E., & Choong, F. X. (2023). Fluorescent optotracers for bacterial and biofilm detection and diagnostics. Science and Technology of Advanced Materials, 24(1), 2246867. https://doi.org/10.1080/14686996.2023.2246867
-
Coppens, B., Belpaire, T. E. R., Rí Pe, J., Steenackers, H. P., Ramon, H., & Smeets, B. (2023). Anomalous diffusion of nanoparticles in the spatially heterogeneous biofilm environment. iScience, 26, 106861. https://doi.org/10.1016/j.isci.2023.106861
-
Lorenz, K., Preem, L., Sagor, K., Putrinš, M., Tenson, T., & Kogermann, K. (2023). Development of In Vitro and Ex Vivo Biofilm Models for the Assessment of Antibacterial Fibrous Electrospun Wound Dressings. Molecular Pharmaceutics, 20(2), 1230–1246. https://doi.org/10.1021/acs.molpharmaceut.2c00902
2022
-
Koch, M., Palarie, V., Koch, L., Burkovski, A., Zulla, M., Rosiwal, S., & Karl, M. (2022). Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies. Microorganisms, 10(4). https://doi.org/10.3390/microorganisms10040782
- Kärkkäinen, E., Jakobsson, S. G., Edlund, U., Richter-Dahlfors, A., & Choong, F. X. (2022). Optotracing for live selective fluorescence-based detection of Candida albicans biofilms. Frontiers in Cellular and Infection Microbiology, 12(981454). https://doi.org/10.3389/fcimb.2022.981454
-
Sass, A., Vandenbussche, I., Bellich, B., Cescutti, P., & Coenye, T. (2022). Pellicle Biofilm Formation in Burkholderia cenocepacia J2315 is Epigenetically Regulated through WspH, a Hybrid Two-Component System Kinase-Response Regulator. Journal of Bacteriology, 204(5). https://doi.org/10.1128/jb.00017-22
-
Butina, K., Lantz, L., Choong, F. X., Tomac, A., Shirani, H., Löffler, S., Nilsson, K. P. R., & Richter-Dahlfors, A. (2022). Structural Properties Dictating Selective Optotracer Detection of Staphylococcus aureus. ChemBioChem, 23(11). https://doi.org/10.1002/cbic.202100684
-
Pham, L. H. P., Colon-Ascanio, M., Ou, J., Ly, K., Hu, P., Choy, J. S., & Luo, X. (2022). Probing mutual interactions between Pseudomonas aeruginosa and Candida albicans in a biofabricated membrane-based microfluidic platform. Lab on a Chip, 22, 4349–4358. https://doi.org/10.1039/d2lc00728b
-
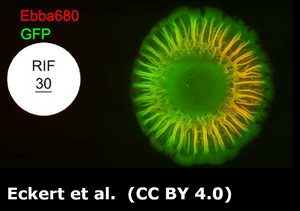
Eckert, J. A., Rosenberg, M., Rhen, M., Choong, F. X., & Richter-Dahlfors, A. (2022). An optotracer-based antibiotic susceptibility test specifically targeting the biofilm lifestyle of Salmonella. Biofilm, 4. https://doi.org/10.1016/j.bioflm.2022.100083
2021
-
Rodríguez-Rojas, A., Baeder, D. Y., Johnston, P., Regoes, R. R., & Rolff, J. (2021). Bacteria primed by antimicrobial peptides develop tolerance and persist. PLoS Pathogens, 17(3), 1–30. https://doi.org/10.1371/JOURNAL.PPAT.1009443
-
Merkl, P., Aschtgen, M. S., Henriques-Normark, B., & Sotiriou, G. A. (2021). Biofilm interfacial acidity evaluation by pH-Responsive luminescent nanoparticle films. Biosensors and Bioelectronics, 171(October 2020), 112732. https://doi.org/10.1016/j.bios.2020.112732
-
Choong, F. X., Huzell, S., Rosenberg, M., Eckert, J. A., Nagaraj, M., Zhang, T., Melican, K., Otzen, D. E., & Richter-Dahlfors, A. (2021). A semi high-throughput method for real-time monitoring of curli producing Salmonella biofilms on air-solid interfaces. Biofilm, 3(September), 100060. https://doi.org/10.1016/j.bioflm.2021.100060
Terms & Conditions
For detailed information on Terms & Conditions, please click the link below.
Privacy Policy
To read more how we handle personal information, please click the link below.
Support
If you have any questions related to our products or services, please contact us.