Aggregated α-synuclein is part of the pathology of Parkinson’s disease and dementia with Lewy bodies, but why these oligomeres and fibrils aggregate as part of these diseases - or how to stop it - is unknown. In a study published in Nature Chemical Biology, Aaron Balana and colleagues investigated the effects of the post-translational modification O-GlcNAc (an intracellular form of glycosylation) of α-synuclein monomers. Using advanced structural tools such as cryogenic electron microscopy, they demonstrate that O-GlcNAcylation makes α-synuclein adopt a distinct fibril structure that reduces its seeding capacity - suggesting that it is less pathological.
They found that this modified α-synuclein amyloid strain forms slower, spreads less efficiently, and is less harmful in both cultured neurons and rodent models. These data suggest a potentially protective role for glycosylation in neurodegenerative disease. In this study they used Amytracker 680 to visualize and quantify the α-synuclein aggregates with high specificity. Amytracker allowed them to directly assess the effect of glycosylation on the fibril formation and to show that less pathological inclusions were formed in neurons from the O-GlcNAcylated α-synuclein fibrils. These insights pave the way for future research and therapeutic strategies aimed at reducing amyloid pathogenicity as a means to advance diagnostics and treatment for dementia.
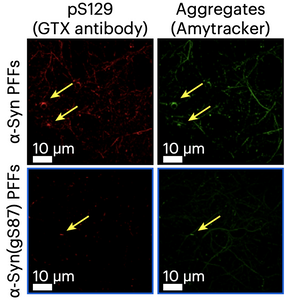
Image: Primary hippocampal mouse neurons were treated with unmodified or glycosylated alpha-synuclein pre-formed fibrils (PFFs). Glycosylation notably reduced the formation of pS129 (red) and Amytracker (green) positive aggregates. Image from Figure 5D by Balana, A.T., Mahul-Mellier, A.L., Nguyen, B.A. et al. (2024)Nat Chem Biol 20, 646–655(CC BY 4.0).
