In the context of neurodegenerative diseases, large, fibrillar amyloid deposits - such as Lewy bodies, Amyloid plaques, and Neurofibrillary tangles - characterize the pathology of the disease but it has recently become evident that small, spherical aggregates below 20 nm in diameter are mainly responsible of the toxic response that results in neurodegeneration. The small size of these aggregates makes their detection difficult, especially within biologically relevant environments. To tackle this issue, Michael Morten and their colleagues at the Imperial College in London have developed a method for single-molecule localization microscopy (SMLM) using aggregate-activated fluorophores. Initially, they benchmarked Alexa647, Thioflavin T, Proteo-Stat and Amytracker 630 staining of aggregates assembled from recombinant α-Synuclein (α-Syn) using standard TIRF imaging. Since Thioflavin T lacked in brightness, it was imaged with 6x more laser power (60 mW) compared to the other fluorophores (10 mW). Exploring the relationship between the total fluorescence intensity detected from each single aggregate versus its size, the researchers found that Proteo-Stat and Amytracker 630 demonstrated greater fluorescence intensities with aggregate size compared to Thioflavin T and even Alexa647, which is due to higher densities of fluorophores bound to each aggregate. This highlights the advantages of aggregate-activated fluorophores over the Alexa647-conjugation approach. Due to the Abbe diffraction limit, determining the size of aggregates <200 nm is challenging. Therefore, the researchers used SMLM to characterize the structural features of aggregates in finer detail. They found that Amytracker 630 is suitable for quantitative SMLM imaging of aggregates at circa 4 nm precision and enables sensitive and quantitative detection of aggregate species down to approximately 10 nm in size in live cells and ex vivo brain tissue.
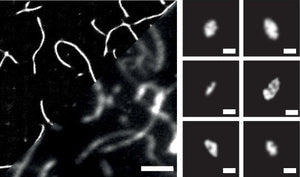
Image: Amytracker 630 labeled recombinant α-Syn aggregates. Left: imaged by SMLM reconstruction and conventional diffraction-limited TIRF (2 µm scale bar). Right: Smaller aggregates detected using SMLM (0.1 µm scale bar). Image from Figure 2D by Morten, M.J. et al. (2022) PNAS 119(41), e2205591119 (CC BY 4.0)).
An advantage of using Amytracker 630 compared to the other fluorophores was its compatibility with live cell imaging. When incubated with recombinant α-Syn, Amytracker 630 staining of live HEK293A cells with GFP-tagged proteasomes revealed that the plasma membrane effectively prevents aggregates >450 ± 60 nm from entering the cell, and that internalized aggregates are quickly surrounded by proteasomes, suggesting that proteostasis mechanisms respond promptly to proteotoxicity. Finally, the size of the membrane-penetrating aggregates detected by Amytracker 630 was shown to correlate with cytotoxicity. When the cytotoxicity assay was performed with aggregates from recombinant α-Syn as well as brain derived aggregates from Parkinson’s Disease and Lewy Body Dementia, it was shown that aggregates differ in toxicity depending on the pathology they originated from. Taken together, the study by Morten et al. is an impressive example of technology development directly benefiting the medical field by providing important clues about the relationship between aggregate size and toxicity.
