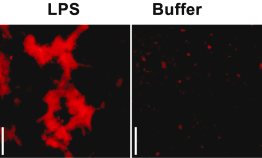
Lipopolysaccharide (LPS) is a cell envelope glycolipid produced by most gram-negative bacteria. When LPS is recognized by Toll-like receptor 4 (TLR4) an innate host-response to bacterial infection is triggered. To achieve a robust antibacterial response while maintaining control of inflammatory processes, LPS has to be cleared from the infection site. A group of researchers around Prof. Artur Schmidtchen from the Division of Dermatology and Venereology at Lund University have investigate the mechanism of LPS clearing from wounds and shown that addition of LPS or bacteria to acute wound fluid (AWF) leads to precipitation of proteins mediating LPS aggregation and scavenging. In a recent study, Petrlova et al. sought to define the LPS interactome in AWF and investigate the functional consequences of LPS aggregation. The researchers use Amytracker 680 to confirm the presence of protein aggregates in AWF treated with high concentrations of LPS (Image: Acute wound fluid incubated with LPS from E.coli or Buffer (negative control) labelled with Amytracker 680 and imaged using fluorescence microscopy. Image from Figure 2D, Petrlova, J. et al. (2023) iScience, 26(10), 107951 (CC BY 4.0).
Using Mass Spectroscopy and western blot, the researchers show that wound-fluid aggregates contain not only thrombin but also a multitude of other proteins, including coagulation factors, annexins, histones, antimicrobial proteins/ peptides, and apo-lipoproteins with many of them also being present in plaques from patients with systemic- or neurodegenerative amyloidoses. To study the functional effect of LPS induced aggregation in acute wound fluids, the researchers performed experiments with reporter monocytes and mice that produce a quantifiable response upon TLR4 activation. These experiments showed that wound fluid inhibits the inflammatory cascade induced by LPS, both in cells and in vivo. While presenting evidence of amyloid formation in a functional context, the study provides another puzzle piece linking amyloid formation to inflammation. Understanding the functional aspect of amyloid formation might pave the way for innovative strategies to mitigate the detrimental effects of amyloid diseases.
