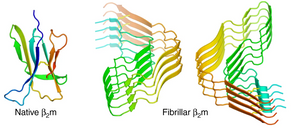
Multiple myeloma is a complex B-cell malignancy characterised by the accumulation of malignant plasma cells. Progression of multiple myeloma is related to dysregulated inflammatiory processes; especially leucocytes and tumor-associated macrophages (TAMs) are central during the initiation and progression of the disease and high concentration of TAMs often relates to drug resistance and fast proliferation. TAMs produce proinflammatory cytokines whose production is controlled carefully in the cell under normal circumstances. The production and release of proinflammatory cytokines is controlled by nod-like receptor NLRP3. While the activation process of NLRP3 is poorly understood, it has been found that, among other triggers, endogenous amyloid fibrils activate NLRP3. In a study published in the journal Immunity, Hofbauer et al. from University Hospital Erlangen in Germany aimed to understand the role of amyloid fibrils in the activation of NLRP3. For this, they used Beta-2-microglobulin (b2m) which is well known for amyloid aggregation propensity. B2m is a small non-glycosylated protein and universally expressed in all nucleated cells. Interestingly, b2m concentration is known to increase in myelomas and is correlated with a poor prognosis. However, the molecular mechanism behind this is not known. The researchers used Amytracker 480 to verify the formation of b2m amyloids in macrophages. Since Amytracker 480 is non-toxic and cell-permeant (Macrophages were washed in PBS and incubated with Amytracker 480 (1:1000) for 1h at 37 °C), amyloid presence in macrophages was quantified using flow cytometry and Amytracker fluorescence was used as an indicator for presence of amyloids. The researchers found that the NLRP3 inflammasome is activated after phagocytosis of β2m and that internalised β2m aggregates into amyloid fibrils under the acidic phagosomal conditions, which results in lysosomal swelling and damage. They further demonstrate that the β2m-triggered NLRP3 activation in TAMs results in the release of proinflammatory cytokines and in turn favours the growth and severity of Multiple Myeloma. These findings provide a strong indication that dysregulated inflammatory response is a driver of disease in Multiple Myeloma and that inhibition of NLRP3 represents a potential therapeutic approach.
Image: The structure of β-2-microglobulin in its native and fibrillar states. Image from Figure 4 AB by Iadanza, M.G., Silvers, R., Boardman, J. et al. (2018) Nature Communications, 9(1), 4517 (CC BY 4.0).
