Biofilms form when bacteria colonies build a communal extracellular matrix polymeric substances. This shield provide both structural integrity and protection against antimicrobial treatments. This makes biofilm-associated infections particularly difficult to eradicate and drives the development of novel strategies to overcome biofilm-mediated resistance.
One promising approach involves the use of nanoparticles (NPs) engineered to carry antimicrobial agents. However, the effectiveness of these NPs depends on their ability to penetrate and distribute throughout the biofilm — a process influenced by complex interactions between NPs and the biofilm’s structure.
In a recent publication, Bart Coppens and Tom E.R. Belpaire from Bart Smeets' group investigated how different biofilm architectures impact NP distribution. Using Salmonella typhimurium as a model system, they cultivated biofilms under nutrient-rich and nutrient-poor conditions to induce structural variation. To visualise the biofilm matrix, the team employed EbbaBiolight 680, which was added directly to the live cultures. After 48 hours of growth, the biofilms were imaged using confocal microscopy.
They found that biofilms grown in nutrient-poor media were thinner compared to those grown in nutrient-rich conditions. When NPs were added, their distribution differed markedly depending on the biofilm’s architecture. In thicker, nutrient-rich biofilms, NPs were largely confined to the upper layers, leaving bacteria deeper in the matrix untouched. In contrast, thinner biofilms allowed for more uniform NP penetration, demonstrating that the extracellular matrix behaves as an absorbing surface, selectively filtering NPs and impeding deeper diffusion.
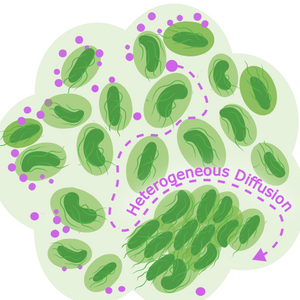
Notably, the study also uncovered significant spatial variation in biofilm viscosity. Viscosity was highest near bacterial cells, contributing to a “sieve-like” effect that results in particle accumulation at the periphery and limited access to the biofilm core. This heterogeneous diffusion landscape plays a crucial role in shaping NP distribution.
This work provides important insight into how biofilm architecture can hinder nanoparticle-based therapies — and highlights the value of EbbaBiolight 680 in uncovering structural features that influence treatment outcomes.
Image: Biofilm distribution (Light green) with pockets of higher viscosity surrounding cells (dark green) creates heterogeneous diffusion of particles, regardless of type i.e; nutrients, antibiotics or other environmental molecules. Image courtesy of Ebba Biotech summarising findings from Coppens et al. (2023). iScience, 26, 106861 (CC-BY-NC-ND)
