Monitoring curli production in liquid culture using EbbaBiolight
This protocol describes how to monitor kinetics of Salmonella extracellular matrix (curli) production in liquid culture. The method described here is based on Choong et al. (2016) npj Biofilms and Microbiomes, 2, 16024 where isogenic mutants of S. Enteritidis were used to identify the extracellular matrix components curli and cellulose as targets for optotracer binding. When used as described, EbbaBiolight does not label Salmonella cell wall and does not influence biofilm formation.
Materials:
Equipment:
Assay Procedure:
Note: When adapting this technique, please make sure to include relevant controls to verify that EbbaBiolight does not affect biofilm formation, to confirm curli as EbbaBiolight binding target and to exclude pH effects. Please be aware that fixation might alter the staining pattern of EbbaBiolight.
Materials:
- EbbaBiolight
- Growth medium
- Bacteria on standard culture plate
- 96-well plate (round bottom) with cover
- Deionized water
Equipment:
- Incubator (28°C)
- Shaking Incubator (37°C)
- Fluorescence plate reader
Assay Procedure:
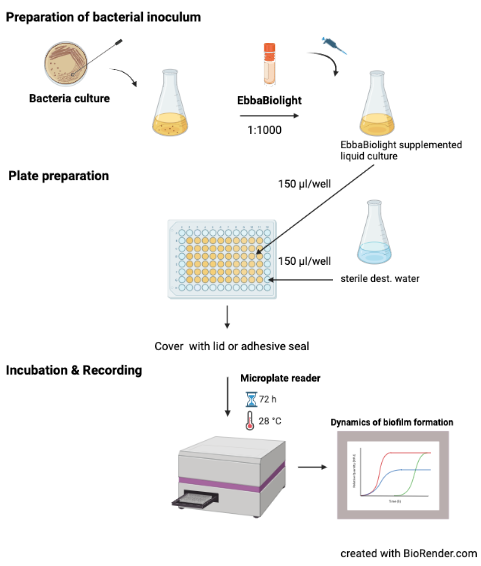
- Prepare bacterial inoculum:
- Pick a colony from a standard culture plate.
- Transfer colony to growth medium.
- Prepare an overnight or exponential culture under continuous shaking at 37°C.
- Dilute bacterial culture 1:100 in fresh growth medium.
- Add EbbaBiolight (1:1000) and mix gently.
- Prepare 96-well plate:
- Use 100-200 µl liquid culture supplemented with EbbaBiolight per well.
- Fill unused wells with sterile water to avoid drying during incubation.
- Seal the plate with a cover.
- Incubation & Readout:
- incubate the 96-well plate directly in the plate reader under static conditions.
- Record excitation- and emission at regular time points during biofilm growth.
Optotracing with EbbaBiolight
EbbaBiolight fluorescent tracer molecules are optotracers. Unlike conventional fluorescent dyes, optotracers bind promiscuously to a range of targets with repetitive motifs. EbbaBiolight has been shown to bind to curli and cellulose in Salmonella extracellular matrix [1,2], peptidoglycan and lipoteichoic acids in the cell envelope of Staphylococci [3], β-glucans from S. cerevisiae and Chitin in C. albicans [4]. Upon binding, the fluorescence intensity of the optotracer increases. This property makes it possible to use EbbaBiolight for live fluorescent tracking of microorganisms, without the need to wash away unbound molecules. It is possible to read out fluorescence intensity at the emission maximum (Emmax) when excited at or close to the excitation maximum (Exmax). This is useful for microscopy or fluorescence spectroscopy when straight-forward data analysis is required. Yet, due to the unique properties of the optotracers, a unique optical fingerprint is produced reflecting the specific nature of the target (sample composition) and environment (pH, osmolarity, polarity of the medium). This means that depending on the specific properties of the sample, Exmax or Emmax can shift, or the appearance of double peaks or shoulders might indicate binding to multiple targets. We therefore recommend acquiring fluorescence excitation and emission spectra whenever possible within experimental limitations. EbbaBiolight excitation- and emission spectra can be accessed here.
| Exmax | Emmax | Excitation spectrum (detect at Emmax) | Emission spectrum (excite at Exmax) | Recommended filter-sets |
|
|---|---|---|---|---|---|
| EbbaBiolight 480 | 420 nm | 480 nm | 300 - 450 nm | 450 - 800 nm | DAPI |
| EbbaBiolight 520 | 460 nm | 520 nm | 300 - 490 nm | 490 - 800 nm | FITC, GFP |
| EbbaBiolight 540 | 480 nm | 540 nm | 300 - 510 nm | 510 - 800 nm | FITC, GFP, YFP |
| EbbaBiolight 630 | 520 nm | 630 nm | 300 - 600 nm | 550 - 800 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
| EbbaBiolight 680 | 530 nm | 680 nm | 300 - 650 nm | 660 - 800 nm | PI, mCherry, Cy3.5 |
Read More:
- Choong FX et al. (2016) Real-Time optotracing of curli and cellulose in live Salmonella biofilms using luminescent oligothiophenes. npj Biofilms and Microbiomes, 2, 16024
- Choong FX et al. (2021) A semi high-throughput method for real-time monitoring of curli producing Salmonella biofilms on air-solid interfaces. Biofilm, 3, 100060
- Butina K. et al. (2020) Optotracing for selective fluorescence-based detection, visualization and quantification of live S. aureus in real-time. npj Biofilms and Microbiomes, 6(1), 35
- Kärkkäinen, E. et al. (2022) Optotracing for live selective fluorescence-based detection of Candida albicans biofilms. Frontiers in Cellular and Infection Microbiology, 12, 2235-2988
