Carbotrace are optotracers for anatomical mapping of carbohydrate structures in plants and for non-destructive composition analysis of glucans in bio-based materials and biofuels.
The optotracing technology entails the use of structure-responsive fluorescent molecules (optotracers) which become fluorescent when binding to a target. The excitation and emission spectra of the optotracer molecule contain information on the structure of their binding partner and their environment. This spectral fingerprint can be used to identify biomolecules in various types of materials.
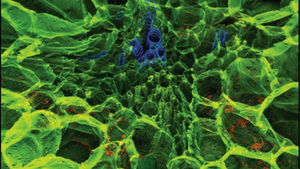
Carbotrace is available in five variants which bind to repetitive motifs in proteins and carbohydrates. It has been shown that Carbotrace 680 binds to homoglucans and heteroglucans with a limited degree of branching. By using the spectral fingerprint from the excitation- and emission spectra, it was shown that the optotracer can differentiate between different types of glycosidic bonds. Using Multi-laser/Multi-detector imaging, non-destructive composition analysis of biomass has been performed such as differentiation of cellulose cell walls and starch granules in fresh potato as well as cellulose and lignin in in various types of biomass. Using fluorescence lifetime imaging in combination with Carbotrace 680, the mechanism of paper ageing was analysed. Further, Carbotrace 680 was used to visualise cellulose content in terrestrial plants (maize root and ulvophyceae) as well as algae and to characterise a cellulose immobilisation matrix in a microfluidic device. Carbotrace 480 was used as a tool for cellulose visualisation and to optimise cellulose extraction from macroalgae.
Carbotrace variant work in a wide range of salt and pH conditions. When the pH is altered during the experiment, pH controls should be included. Carotrace can be used with fluorescence plate readers, fluorescence microscopes and confocal laser scanning microscopes and fluorescence life time imaging.
Store your Carbotracer product in the fridge and use the opened container within 12 months. Carbotrace is for research use only and is not for resale.

Carbotrace Testing Service
Are your interested in composition analysis using Carbotrace, but lack necessary equipment or expertise?
With our Carbotrace Testing Service, we can help you get started!
If you send us your material, we will be able to label your sample with a Carbotrace variant of your choice and acquire data using our protocols and in-house equipment. As a result, you will receive a report including some processed data and all the raw data. Samples are able to be sent back or destroyed upon request when the project is completed.
Please click on the button to fill out an inquiry form.
If you fill out all fields, we will be able to get back to you with a detailed quote for a Carbotrace Testing Service tailored to your needs. If you only fill out your name and email, we will be happy to get in touch to discuss the best way forward.
Fill out Carbotrace Testing Service Request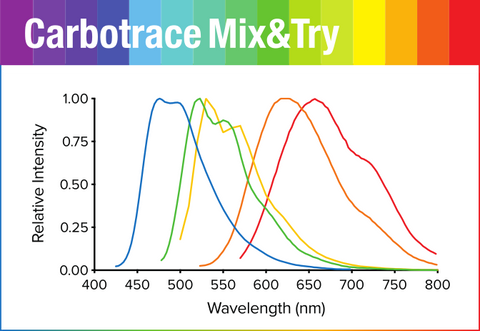
Carbotrace Mix&Try is our recommended option for starting out with using Carbotrace. It contains 10 µL of each variant. Testing each variant will allow you to determine which variant is best suited for your experiments and available instruments.
All Carbotrace variants label repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Labelling of hemicelluloses is weak or absent.
Carbotrace 480 has been used to optimize a sustainable extraction method for cellulose hydrogels derived from microalgae and Carbotrace 680 has been used to image cellulose and starch components in plant cells and differentiate between those components using multi-laser/multi-detector analysis with confocal microscopy. Using a fluorescence spectrophotometer to acquire excitation and emission spectra, a unique spectral signature allows to differentiate between α(1-3), α(1-4), α(1-6), β(1-3), β(1-4) and β(1-6) linked glucans. As such, Carbotrace 680 labels cell wall components such as Cellulose, Chitin, Laminarin and the hemicellulose xyloglucan. Further, Carbotrace 680 has been used to determine relative carbohydrate content and elasticity of cell walls in plant material. Contact us to learn more about applications for Carbotrace.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 480 | 420 nm | 480 nm | DAPI |
| Carbotrace 520 | 460 nm | 520 nm | FITC, GFP |
| Carbotrace 540 | 480 nm | 540 nm | FITC, GFP, YFP |
| Carbotrace 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
| Carbotrace 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |
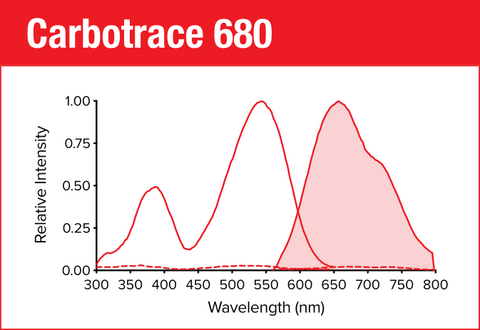
Carbotrace 680 is our red optotracer for labeling repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Generally labelling of hemicelluloses is weak or absent.
Specifically, Carbotrace 680 has been used to image cellulose and starch components in plant cells and differentiate between those components using multi-laser/multi-detector analysis with confocal microscopy. Using a fluorescence spectrophotometer to acquire excitation and emission spectra, a unique spectral signature allows to differentiate between α(1-3), α(1-4), α(1-6), β(1-3), β(1-4) and β(1-6) linked glucans. As such, Carbotrace 680 labels cell wall components such as Cellulose, Chitin, Laminarin and the hemicellulose xyloglucan. Further, Carbotrace 680 has been used to determine relative carbohydrate content and elasticity of cell walls in plant material. Contact us to learn more about applications for Carbotrace 680.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 680 | 530 nm | 680 nm | PI, mCherry, Cy3.5 |
Carbotrace 680 is available in four different formulations (See volumes and prices in the drop-down list below):
- Aqueous: 1 mg/ml solution in ultrapure water. The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
- DMSO: 1 mg/ml solution in DMSO to prevent solvent evaporation. The product should be diluted 1:1000 before use.
- Solid: 1 mg solid lyophilised in a sterile injection bottle.
- Drop&Shine: 5 ml ready-to-use product in mounting medium. Ideal for use in sections. Add a some Drop&Shine and mount your slide to detect biofilms within minutes.
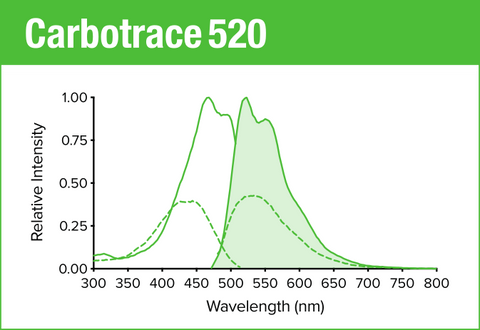
Carbotrace 520 is our green optotracer for labeling repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Generally labelling of hemicelluloses is weak or absent. Contact us to learn more about applications for Carbotrace 520.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 520 | 460 nm | 520 nm | FITC, GFP |
Carbotrace 520 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
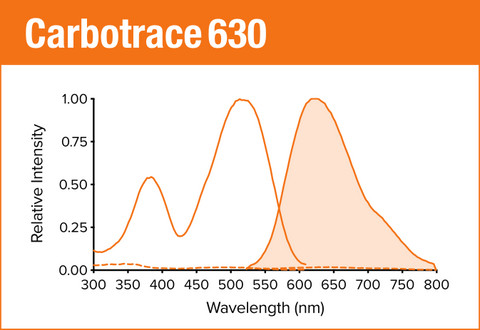
Carbotrace 630 is our orange optotracer for labeling repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Generally labelling of hemicelluloses is weak or absent. Contact us to learn more about applications for Carbotrace 630.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 630 | 520 nm | 630 nm | PI, Cy3, TxRed, mCherry, Cy3.5 |
Carbotrace 630 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
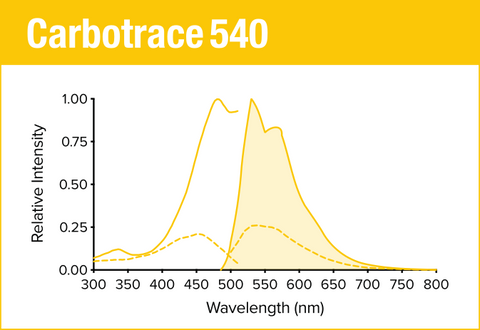
Carbotrace 540 is our yellow optotracer for labeling repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Generally labelling of hemicelluloses is weak or absent.
Specifically, Carbotrace 540 has been used to determine the purity of cellulose extracted from macroalgae by allowing analysis of extracted carbohydrates in their polymeric state. In addition, Carbotrace 540 has shown to bind to the hemicellulose xyloglucan. Contact us to learn more about applications for Carbotrace 540.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 540 | 480 nm | 540 nm | FITC, GFP, YFP |
Carbotrace 540 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
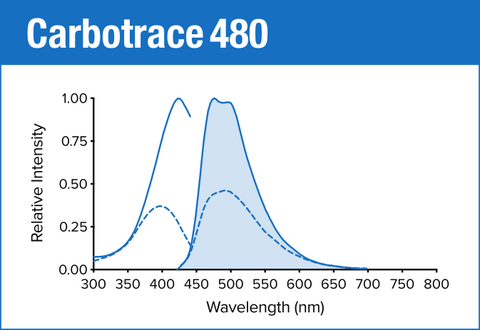
Carbotrace 480 is our blue optotracer for labeling repetitive motifs in proteins and carbohydrates. Generally, Carbotrace labeling is strongest with protein aggregates. Homoglucans like cellulose are labeled reliably, albeit with lower quantum yield. Heteroglucans with low degree of branching like starches are labeled and can be distingiushed by their spectral fingerpint. Generally labelling of hemicelluloses is weak or absent.
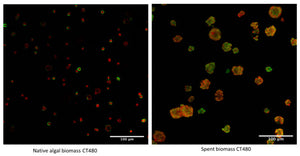
Specifically, Carbotrace 480 has been used to optimize a sustainable extraction method for cellulose hydrogels derived from microalgae. Contact us to learn more about applications for Carbotrace 480.
| Exmax | Emmax | Recommended filter-sets | |
|---|---|---|---|
| Carbotrace 480 | 420 nm | 480 nm | DAPI |
Carbotrace 480 is available as 1 mg/ml solution in ultrapure water (Aqueous) with volumes ranging from 10 - 200 µL (See volumes and prices in the drop-down list below). The product should be diluted 1:1000 before use. To prevent evaporation of the aqueous solvent, close the container carefully after use, spin down liquid and use up small volumes quickly.
Protocol I: Anatomical mapping of cellulose in plant tissues using Carbotrace 680
Read more →
Protocol II: Measurement of cellulose content in a sample using Carbotrace 680
Read more →
Carbotrace as a tool for the study of cellulose oxidation and paper aging
Read more →
Making greener cellulose hydrogel
Read more →
Carbohydrate content and elasticity of cell walls in elongating maize root
Read more →
Breaking barriers in early detection of cancer
Read more →
Carbotrace 540 was used to determine purity of cellulose extracted from sea lettuce
Read more →
Carbotrace-like molecules allow highly detailed visualization of cellulose in plant cells
Read more →
Carbotrace 680 for carbohydrate anatomical mapping
Read more →
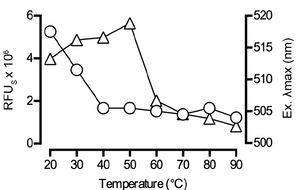
Monitoring heat-induced swelling of Starch granules
Read more →
Carbotrace fluorescence spectra
Read more →
Carbotrace 680 for monitoring starch
Read more →
Testimonial - Reina Tanaka
Read more →
Testimonial - Frederik Zitzmann
Read more →
Testimonial - Saga Jakobsson
Read more →
Testimonial - Aristoteles Goes-Neto
Read more →
Testimonial - Ulrica Edlund
Read more →
Testimonial - Ferdinand X. Choong
Read more →
Testimonial - Ulrica Edlund
Read more →
Testimonial - Liudmila Kozlova
Read more →
Testimonial - Tharagan Kumar
Read more →
Incorporating Carbotrace as a problem solver in a biorefinery design process
Read more →
Cellulose in Cancer Diagnostics
Read more →
Spectral identification and recovery of polysaccharides from biorefining
Read more →
Optotracers - multifunctional fluorescent tracers
Read more →
Carbotrace 540 helps to determine purity of plant raw materials
Read more →
Carbotrace-like molecules for visualization of cellulose in plants
Read more →
Carbotrace supports circular economy
Read more →
We named our Carbotrace molecules after their peak emission wavelength when they are bound to their target. That means, when Carbotrace is bound to a target, it will emit fluorescence at peak emission indicated by the number associated with its name.
To view the excitation and emission spectra, please select your Carbotrace below :
2024
- Schmidt, Alina E. M., Ferdinand X. Choong, Agneta Richter‐Dahlfors, and Ulrica Edlund. 2024. “Defibrillated Lignocellulose Recovery Guided by Plant Chemistry and Anatomy – a Pioneering Study with Lupinus Angustifolius.” Advanced Sustainable Systemshttps://doi.org/10.1002/adsu.202300632.
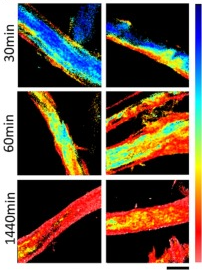
- Ferrara, Vittorio, Valeria Vetri, Bruno Pignataro, Delia Francesca Chillura Martino, and Giuseppe Sancataldo. 2024. “Phasor-FLIM Analysis of Cellulose Paper Ageing Mechanism with Carbotrace 680 Dye.” International Journal of Biological Macromolecules 260 (March): 129452. https://doi.org/10.1016/j.ijbiomac.2024.129452.
2023
- Holzinger, Andreas, Niklas Plag, Ulf Karsten, and Karin Glaser. 2023. “Terrestrial Trentepohlia Sp. (Ulvophyceae) from Alpine and Coastal Collection Sites Show Strong Desiccation Tolerance and Broad Light and Temperature Adaptation.” Protoplasma. https://doi.org/10.1007/s00709-023-01866-2.
2022
- Zitzmann, Frederik L., Ewan Ward, and Avtar S. Matharu. 2022. “Use of Carbotrace 480 as a Probe for Cellulose and Hydrogel Formation from Defibrillated Microalgae.” Gels 8 (June): 383. https://doi.org/10.3390/gels8060383.
- Petrova, Anna, Gusel Sibgatullina, Tatyana Gorshkova, and Liudmila Kozlova. 2022. “Dynamics of Cell Wall Polysaccharides During the Elongation Growth of Rye Primary Roots.” Planta 255 (May): 108. https://doi.org/10.1007/s00425-022-03887-2.
2020
- Petrova, Anna, Tatyana Gorshkova, and Liudmila Kozlova. 2020. “Gradients of Cell Wall Nano-Mechanical Properties Along and Across Elongating Primary Roots of Maize.” Journal of Experimental Botany. https://doi.org/10.1093/jxb/eraa561.
- Kumar, Tharagan, Ruben R G Soares, Leyla Ali Dholey, Harisha Ramachandraiah, Negar Abbasi Aval, Zenib Aljadi, Torbjörn Pettersson, and Aman Russom. 2020. “Multi-Layer Assembly of Cellulose Nanofibrils in a Microfluidic Device for the Selective Capture and Release of Viable Tumor Cells from Whole Blood †.” Nanoscale. https://doi.org/10.1039/d0nr05375a.
2019
- Choong, Ferdinand X., Linda Lantz, Hamid Shirani, Anette Schulz, K. Peter R. Nilsson, Ulrica Edlund, and Agneta Richter-Dahlfors. 2019. “Stereochemical Identification of Glucans by a Donor–Acceptor–Donor Conjugated Pentamer Enables Multi-Carbohydrate Anatomical Mapping in Plant Tissues.” Cellulose 26: 4253–64. https://doi.org/10.1007/s10570-019-02381-5.