The Secrets of Utricularia: Nature’s Fastest Traps
Utricularia species, also known as bladderworts, are aquatic carnivorous plants. They have evolved one of the fastest movement mechanisms in the plant kingdom that allows them to trap and consume zooplankton, tiny insects, or algae. When an unsuspecting prey touches the sensory hairs on the trap door the bladder instantly generates negative pressure, sucking in water along with the prey. Scientists are still uncertain about the mechanism of resetting its trap. Some suggest that internal glands actively absorb and expel water, while others propose that specialized external glands play a crucial role in water expulsion.
A study published in the International Journal of Molecular Sciences takes a deeper look into the external glands. By analyzing - using Carbotrace 680 - the structural layers of their cell walls, researchers have uncovered variations in homogalacturonans and hemicelluloses. These differences could be the key to understanding how bladderworts efficiently reset their traps and prepare for their next meal. The team found that the outermost layer of the gland cells of Utricularia dichotoma lacked arabinogalactan proteins, while the deeper layers were rich in them. The presence of these proteins correlates with the presence of hemicelluloses, which regulate cell wall expansibility and cell-to-cell adhesion, and suggests a functional specialization in water transport and secretion. This innovative use of Carbotrace highlights its potential for advanced plant biology research, helping scientists better understand how plants regulate fluid movements through cell wall composition.
This research not only sheds light on the biomechanics of one of nature’s fastest predators but also offers valuable insights into plant cell wall specialization and fluid transport.
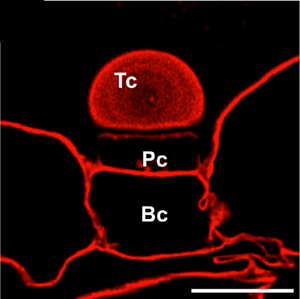
Image: The terminal cell (Tc), pedestal cell (Pc), and basal cell (Bc) of the external gland in Utricularia dichotoma were stained with Carbotrace 680 (in red). The lack of staining in the latera walls of the pedestal cell (Pc) and the cell wall of the terminal cell (Tc) is probably related to the presence of cutin in these regions. Scale bar: 10 µm. Image adapted from Figure 6A by Płachno, B. et al. (2024) nternational Journal of Molecular Sciences, 25(23), 13124. (CC BY 4.0).
Read More:
A sustainable solution for transforming food waste into high-value hydrogels
Researchers from the University of York focused on creating hydrogels from blackcurrant pomace, a residue from the food supply chain. They aimed to explore sustainable methods for producing valuable biopolymers like cellulose through microwave-assisted hydrothermal fractionation, thus reducing waste. The team first isolated pectin at various temperatures, then used a second microwave treatment to extract defibrillated cellulose from the remaining pomace residues. They assessed the cellulose’s ability to form hydrogels, a process enhanced through further bleaching steps.
To visualise the cellulose and ensure effective extraction, the researchers utilised Carbotrace 480 binding to cellulose, allowing them to observe cellulose distribution via confocal laser scanning microscopy (CLSM). The images revealed a strong interaction between Carbotrace and cellulose, which helped confirm the removal of impurities like lignin from the samples.
In conclusion, the study demonstrated that blackcurrant pomace could be effectively processed into hydrogels with high water-holding capacity. The use of microwave hydrothermal treatment and Carbotrace for visualisation provided valuable insights into the cellulose purification process, offering potential applications in pharmaceuticals, packaging, and other industries.
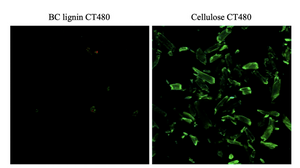
Image: Carbotrace 480 selectively stains cellulose (shown in green, right) but does not bind to BC lignin (left), as demonstrated by confocal laser scanning microscopy (CLSM). This clear distinction highlights Carbotrace 480's specificity for cellulose in the sample. Image from Figure 10 by Inthalaeng et al 2023, Gels, 9 (9), 674 (CC BY 4.0).
Read More:
Carbotrace for differentiation between lignin and cellulose in renewable resources
A study by researchers from KTH explores Lupinus angustifolius (a type of lupin) as a sustainable source of lignocellulose materials. In order to reduce reliance on forest wood and utilise agricultural residues to produce microfibrillated cellulose (MFC), the team analysed the anatomical structure and chemical composition of the plant, classifying parts into stems, roots, seed pods, and seeds and developed a mild extraction process using alkaline treatment to create lignin-containing MFC (L-MFC) from non-edible residues.
The researchers employed several methods to track changes during extraction: they used scanning electron microscopy (SEM) and Fourier-transform infrared spectroscopy (FTIR) to examine the plant's morphology and chemical transformations. Using Carbotrace 680 allowed them to map cellulose distribution in native tissues and throughout the extraction. By soaking tissue sections in Carbotrace and analysing them under a confocal microscope, they visualised the spatial relationships between cellulose and lignin in real-time; this provided critical insights into the distribution of biomacromolecules across various tissues.
The study concluded that Lupinus angustifolius is a viable alternative source for lignocellulose materials. The mild extraction process successfully separated cellulose while preserving lignin, which enhanced the strength and thermal properties of the final product; Carbotrace enabled precise, non-invasive visualization of cellulose, guiding efficient lignocellulose recovery throughout the process. This research highlights the potential of agricultural residues for producing bio-based materials, offering a sustainable alternative to forest-derived resources.
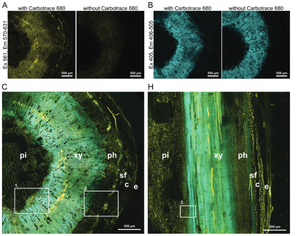
Image: Confocal fluorescence microscopy images of Lupinus angustifolius tissues stained with Carbotrace 680 to highlight the distribution of lignin and cellulose. Panels (A) and (B) display cross-sections with and without Carbotrace 680. In (A), excitation at 561 nm (emission 570-631 nm) highlights cellulose in yellow fluorescence, while the control shows no signal, confirming the stain’s specificity. Panel (B) shows lignin in blue (excitation 405 nm, emission 406-505 nm), for which Carbotrace does not stain, showing there is autofluorescence with and without the Carbotrace. Panels (C) and (H) illustrate cross-sections and longitudinal sections of the stem, showing the distribution of lignin and cellulose in tissues like xylem (xy), phloem (ph), and pith (pi). Insets in (C) and (H) provide detailed views of these regions. Image from Figure 2A,B,C and H by Schmidt et al 2024, Advanced Sustainable Systems, 8, 2300632 (CC BY 4.0).
Read More:
Carbotrace as a tool for the study of cellulose oxidation and paper aging
Since its invention, paper has skyrocketed technological advances as a key medium for recording thoughts and ideas. However, over time paper ages and oxidation leads to loss of strength and renders sheets of paper brittle. Therefore, the study of paper aging becomes relevant not only for the preservation of historical and artistic values, but also because competition from electronic media and increasing demands from customers force the pulp and paper industry to improve its competitive edge by optimizing production processes and paper quality. In a recent publication from the Departments of Physics and Chemistry as well as Biological, Chemical and Pharmaceutical Sciences at the University of Palermo, Ferrara et al. used fluorescence-lifetime imaging microscopy (FLIM) to study aging of cellulosic material. The researchers leveraged the structure-dependent photo-physical properties of Carbotrace 680 to track aging-induced chemical alterations in cellulose fibers. Paper aging was simulated by treating cellulose fibers with potassium metaperiodate which reacts with cellulose to yield the oxidized derivative known as dialdehyde cellulose in a controlled Malaprade reaction. Paper acidification and carbonyl content were studied with conventional methods such as pH measurement, a redox colorimetric method (TTC assay) and crystallinity analysis using X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR) techniques. As an alternative, novel method, steady-state fluorescence and phasor-FLIM analysis of paper oxidation were performed using Carbotrace. Confocal microscopy of cellulose fibers labeled with Carbotrace 680 before and after aging revealed morphological differences in the aged fibers. Strikingly, the fluorescence emission spectrum of Carbotrace 680 showed a blue-shift of the emission maximum and a narrowing of the spectrum in aged paper related to alterations in the chemical environment of the dye. The data indicate that Carbotrace 680 fluorescence is sensitive to paper changes induced by oxidation, and showcase Optotracing as a useful technique for screening physicochemical properties of cellulosic materials. Using an advanced method called Fluorescence Lifetime Imaging (FLIM), it is possible to map aging-induced alterations at the sub-micrometric scale. With FLIM, a distribution of fluorescence lifetimes is observed. Using phasor analysis fluorescence lifetime distributions can be compared, revealing striking differences between fluorescence lifetimes of untreated and aged paper. The high-resolution data show that the paper fibers undergo oxidation in a non-uniform way, with the outer portions of the fiber oxidized at the beginning of the process, followed by a complete oxidation of the fiber also in the inner part within 24 h. The study by Ferrara et al. paves the way for use of novel technologies like FLIM and Optotracing in pulp and paper industry that are critical to improve sustainability while maintaining quality.
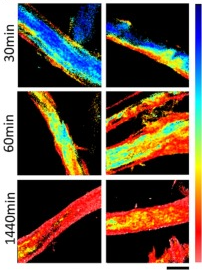
Image: Phasor-FLIM images illustrate kinetics of paper oxidation during 30, 60 and 1440 min treatment with metaperiodate showing the change of the Carbotrace 680 lifetime (blue: 0.10 ns, red: 2.94 ns) during the oxidation process (scale bar: 20 µm). Image from Figure 7C by Ferrara et al. (2024) International Journal of Biological Macromolecules 260(2), 129452 (CC BY 4.0).
Read More:
Making greener cellulose hydrogel
The use of Microalgae as a renewable resource has attracted large interest due to their potential for use in the renewable energy, biopharmaceutical, and nutraceutical industries. Microalgae are renewable, sustainable, and economical sources of biofuels, bioactive medicinal products, and food ingredients. Recently, researchers around Professor Avtar Singh Matharu from the Green Chemistry Centre of Excellence in York, United Kingdom have developed a green process to produce cellulose hydrogel using native and spent microalgae. Cellulose extraction from microalgae without any pre-treatment is difficult, since the microalgal cell wall is very thick and rigid, but the researchers have developed a protocol for Microwave-assisted extraction (MAE) which efficiently disrupts algal cell walls aiding the production of defibrillated celluloses that are then further processed to form cellulose-based hydrogels. Cellulose hydrogel is an emerging biomaterial with many different applications such as use as cosmetics, lubricants and within medical biotechnology.
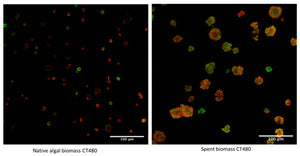
In a recent publication, the researchers at the Green Chemistry Centre of Excellence with Frederick L. Zitzman as lead-author used Carbotrace 480 to benchmark the MAE process and investigate why only MAE at 220°C yields high-quality cellulose hydrogels. Zitzmann et al. performed MAE at different temperatures with native and spent microalgae and used Carbotrace 480 to visualize cellulose in all samples. Carbotrace 480 has its emission maximum in the region of 480 nm. Upon binding to cellulose, the emission peak shifts approx. 20 nm and the intensity in emission increases by around a factor of two. Its spectral properties make Carbotrace 480 the ideal probe for investigating cellulose in microalgae since it allows for spectral unmixing with the autofluorescence of the biomass peaking at 670 nm. Carbotrace 480 shown to be useful to determine differences in morphology between native and spent biomass showing the native biomass as an array of microalgae cells, each with a ring of cellulose encapsulating the cells and the spent biomass as an array of smudged and smashed cells with irregular shapes and a more even distribution of cellulose. Carbotrace 480 also showed clearly an increase in cellulose fluorescence when MAE was performed at higher temperatures in native and spent biomass (see figure). The Carbotrace 480 signal correlated very well with the observed capability for hydrogel formation. Therefore, Carbotrace has proven to be exceptionally suited to aid the exploitation of potential renewable resources and the development of greener processes for hydrogel production.
Image: Carbotrace 480 labeling cellulose in biomass (green) with biomass autofluorescence (red). From Zitzmann, F.L. et al. (2022) Gels, 8(6), 383. (CC BY 4.0)).
Read More:
Carbohydrate content and elasticity of cell walls in elongating maize root
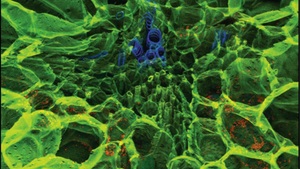
Investigation of plant growth and development is now more than ever an important field of study following the many challenges faced in agriculture. Accurate methods and computational models of plant cell- and organ change during growth are therefore essential to tackle problems like increased food demand in the wake of climate change. Researchers at Kazan Institute of Biochemistry and Biophysics aimed to determine if the mechanical properties of root tissues can control growth. Since growth rate and direction is determined by mechanical properties of plant cell walls, they used an approach based on atomic force microscopy (AFM) to study cell wall elasticity and turgor pressure in elongating primary root tissues of maize. They determined the elasticity of maize root cell walls in the four root development zones called meristem, early elongation-, active elongation-, and late elongation zone. Interestingly, they found that cell walls in root tissues show different patterns of elasticity along the length of the root. Some cell walls have increased stiffness only in the late elongation zone (AAAB type), others had increased stiffness in meristem and late elongation zones (BAAB type) and again others showed the same pattern, but the stiffness was even more increased in the late elongation zone than in the meristem (BAAC type). To understand if there is a link between the cell wall’s elasticity and its polysaccharide composition, the researchers coupled AFM analysis with fluorescent labelling of cell wall polysaccharides. Carbotrace 680 was used for detection and quantification of cellulose.
Image: Carbotrace 680 labelling cellulose in maize root. Image courtesy of Anna Petrova. Click here to read more about the author's experience with Carbotrace 680) along with fluorescent labelled antibodies.
The signal intensity of Carbotrace 680 and the polysaccharide-binding antibodies was plotted against the elasticity in the different developmental zones along the length of the root. In contradiction to previous studies, no correlation was observed. The presence of the polysaccharides studied could therefore not be used to predict the elastic moduli of cell walls. However, by normalizing the fluorescence intensity of the fluorescent antibodies binding to hemicelluloses to that of Carbotrace 680 binding to cellulose, they revealed that a reduction in the degree of homogalacturonan methylation was coupled with a transition from division to elongation and the overall decrease in the elastic modulus of cell walls between the meristem and early elongation zone of maize root. Further, the authors were able to show that cellulose and galactoxyloglucan contents in cell walls might be important for growth patterns in developing roots. Understanding how environmental factors can influence root growth and, eventually, the ability to predict and engineer certain growth properties might be an essential step to face current and future challenges. There is a high demand for increased biomass to feed the world's population considering the effects of global warming and following a global initiative to replace fossil fuels with renewable resources. At Ebba Biotech, we are extremely proud that Carbotrace contributes to research looking into carbohydrate content of plant cell walls on a subcellular level.
Read More:
Breaking barriers in early detection of cancer
The ability to detect and isolate circulating tumor cells from blood could be a life-saving diagnostic tool for early detection of cancer and downstream analysis of tumor growth and metastasis. Researchers from the lab of Aman Russom at the Royal Institute of Technology (KTH) in Sweden have engineered a microfluidic device which captures circulating tumor cells from whole blood using antibodies immobilized on a cellulose nanofibril substrate. Using the device, circulating tumor cells were enriched and captured from whole blood. Importantly, 94.5 % of the captured tumor cells could be released from the device and isolated for downstream analysis. The innovation making such a high release efficiency possible lies in a layer-by-layer assembly of cellulose nanofibrils which serves as substrate for the immobilized antibodies. Using Carbotrace 680, the layer-by-layer assembly was carefully tuned to prevent unspecific binding of antibodies and to optimize the release of captured tumor cells during enzymatic degradation of the cellulose substrate. With the help of Carbotrace 680, the researchers were able to visualize the amount of cellulose in the microfluidic channel during the coating procedure and during enzymatic degradation of the cellulose nanofibrils. That means they were in control of the experimental conditions for optimal antibody immobilization and cell release at every step of development. Thus, they were able to determine the optimal number of layers for capturing and releasing cancer cells. The study, published in the journal Nanoscale, represents a real break-through in cancer diagnostics using a microfluidic device. Cellulose nanofibrils are featured as outstanding, bio- degradable material for capture and release of cells in diagnostic microelectromechanical systems (MEMS). Using Carbotrace to visualize the amount of cellulose nanofibril assemblies therefore adds a new level of precision to the engineering and optimization of these devices.
Read More:
Carbotrace 540 was used to determine purity of cellulose extracted from sea lettuce
Researchers from the Royal Institute of Technology and Karolinska Institutet in Sweden reported about an environmentally friendly process to obtain pure cellulose nanofibrils from the green macroalgae ulva lactuca. Cellulose was extracted by sequential treatment with ethanol, hydrogen peroxide, sodium hydroxide, and hydrochloric acid. A mechanical homogenization process was used to disintegrate the cellulose-rich fraction into cellulose nanofibrils. Analysis of the monosugars reveal that the extracted cellulose contains mainly glucose, but also a fraction of xylose. Carbotrace 540 allowed the researchers to analyze the carbohydrates in its polymeric state and was key to uncover that the macroalgae contain a mixture between highly pure and a xylose-glucose polysaccharide, such as xyloglucan.
Read More:
Carbotrace-like molecules allow highly detailed visualization of cellulose in plant cells
A study from 2018 published in Nature Scientific Reports used a Carbotrace-like molecule to visualize the location and structure of cellulose in plant cells. Applying the molecule to thin slices of onion in a simple procedure resulted in highly fluorescent cell walls and allowed to obtain detailed 3D visualization of perforated sieve plates and plasmodesmata in onion cells. The fluorescence of chlorophyll containing chloroblasts and lignin structures were easily distinguished from the fluorescent Carbotrace-like molecule. The researchers found that the molecule bound with a high specificity to β-linked glucans (polysaccharides derived from glucose) with a degree of polymerization > 8. Thus β-linked glucans like cellulose and laminarin were clearly distinguished from α-linked glucans like amylose, amylopectin, glycogen or dextran. For the first time, non-disruptive carbohydrate analysis of glucans can be performed using a specific fluorescent label for β-linked glucans. Many β-linked glucans are medically important and represent drug-targets for antifungal medications. Moreover, extraction techniques for renewable resources rely heavily on the chemical composition of their raw materials. Therefore, Carbotrace and Carbotrace-like molecules represent a quantum leap for bio-refinery of renewable resources and bio-fuels from plant-derived materials.
Read More:
Carbotrace 680 for carbohydrate anatomical mapping
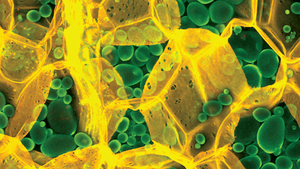
In a research paper published in Cellulose, our structure-responsive optotracer molecule Carbotrace 680 was used to demonstrate the potential of optotracing for carbohydrate anatomical mapping and spectral imaging. As an example - to show the utility and ease of the new technology - Carbotrace 680 was applied to thinly sliced potato samples and then imaged with a fluorescence microscope. Strikingly, Carbotrace 680 bound to cellulose in the cell wall exhibited a unique fluorescent spectrum which was easily separated from the fluorescent spectrum of the molecule when bound to amylose and amylopectin in the potato's starch granules.
Image: The acquired image elegantly shows cell walls in great detail labeled yellow and starch granules labeled green. Evidently, the image made an impression with the editors of the "Cellulose" journal, since it was selected to appear on the cover of the 26(7) edition.
Testing a variety of glucans (polysaccharides made up of glucose) with different types of glycosidic linkages by acquiring the optical spectrum of Carbotrace 680 when bound to the carbohydrate, it was found that Carbotrace 680 differentiates α(1-3), α(1-4), α(1-6), β(1-3), β(1-4) and β(1-6) linked glucans at the molecular level and allows differentiation of cellulose and laminarin from amylose, amylopectin, glycogen and dextran. As starch is an important carrier material for drug formulations, the structure-responsive optotracer Carbotrace 680 was further applied for analyzing heat-induced swelling of starch granules and was proposed as a tool for quality control of starch-containing materials by monitoring its structural changes during production and packaging. The study describes the use of optotracing for anatomical mapping of plant tissue as well as spectral imaging and shows the potential for Carbotrace as an important tool in packaging and production of foods and drugs and in biorefinery applications for renewable materials and biofuels.