Spider silk is an exceptional biomaterial known for centuries and used in medicine as suture threads and wound dressing thanks to its tensile strength, thinness and biocompatibility. Many of the properties of spider silk are a direct consequence of the structure and organization of spidroins, the proteins that make up the fiber. Spidroins contain high fractions of hydrophobic amino acids and repetitive sequences, which shift from an alpha-helical structure in solution to a beta-sheet assembly forming fibrils. This assembly transition is guided by the terminal regions of the spidroid proteins.
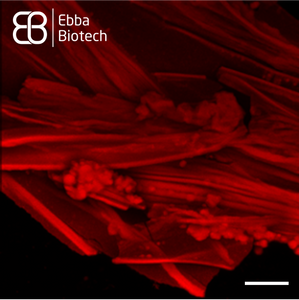
However, natural production of spider silk is unsustainable as spiders produce small amounts. Recombinant spider silk proteins can solve this problem. Using recombinant proteins for synthesis of spider silk is key to producing material in sufficient quantities for impactful applications. They can be designed to form different shapes such as microspheres, membranes and surface coatings based on carefully controlled conditions and additives. Understanding the molecular details of spider silk assembly from recombinant proteins is the main bottle-neck for industry expansion. As Amytracker binds repetitively arranged beta sheets, it is a useful tool to track spider silk assembly. Amytracker can easily be introduced and imaged using fluorescence microscopy with no damage to the silk proteins themselves (see Image). This way, Amytracker can visualise development of these useful shapes and bridge the gap from cutting edge medical research to exciting new industry applications.
Image: Spider silk fibers stained with Amytracker 680 (red). Acquired using confocal fluorescence microscopy with 20X objective lens. Scale bar: 25 µm. Spider silk was graciously provided by Spiber Technologies AB, Sweden. Image ©Ebba Biotech AB.
Read More:
- De Oliveira, D.H. et al. (2024) Structural conversion of the spidroin C-terminal domain during assembly of spider silk fibers. Nature Communications, 15(1), 4670
- Ornithopoulou, E. et al. (2023) Self-assembly of RGD-functionalized recombinant spider silk protein into microspheres in physiological buffer and in the presence of hyaluronic acid. ACS Applied Bio Materials, 6(9), 3696–3705
