Protein aggregation in wound healing
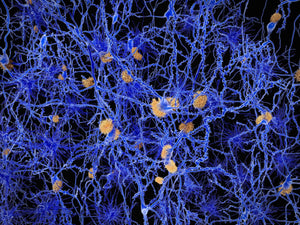
Lipopolysaccharide (LPS) is a cell envelope glycolipid produced by most gram-negative bacteria. When LPS is recognized by Toll-like receptor 4 (TLR4) an innate host-response to bacterial infection is triggered. To achieve a robust antibacterial response while maintaining control of inflammatory processes, LPS has to be cleared from the infection site. A group of researchers around Prof. Artur Schmidtchen from the Division of Dermatology and Venereology at Lund University have investigate the mechanism of LPS clearing from wounds and shown that addition of LPS or bacteria to acute wound fluid (AWF) leads to precipitation of proteins mediating LPS aggregation and scavenging. In a recent study, Petrlova et al. sought to define the LPS interactome in AWF and investigate the functional consequences of LPS aggregation. The researchers use Amytracker 680 to confirm the presence of protein aggregates in AWF treated with high concentrations of LPS (Image: Acute wound fluid incubated with LPS from E.coli or Buffer (negative control) labelled with Amytracker 680 and imaged using fluorescence microscopy. Image from Figure 2D, Petrolova et al. (2023) iScience, 26(10), 107951).
Using Mass Spectroscopy and western blot, the researchers show that wound-fluid aggregates contain not only thrombin but also a multitude of other proteins, including coagulation factors, annexins, histones, antimicrobial proteins/ peptides, and apo-lipoproteins with many of them also being present in plaques from patients with systemic- or neurodegenerative amyloidoses. To study the functional effect of LPS induced aggregation in acute wound fluids, the researchers performed experiments with reporter monocytes and mice that produce a quantifiable response upon TLR4 activation. These experiments showed that wound fluid inhibits the inflammatory cascade induced by LPS, both in cells and in vivo. While presenting evidence of amyloid formation in a functional context, the study provides another puzzle piece linking amyloid formation to inflammation. Understanding the functional aspect of amyloid formation might pave the way for innovative strategies to mitigate the detrimental effects of amyloid diseases.
Read More:
Phase separated α-synuclein is more prone to aggregation
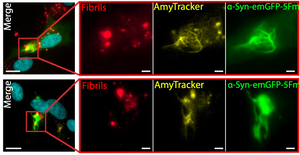
Alpha-synuclein (α-Syn) aggregation hallmarks a group of neurodegenerative diseases known as synucleinopathies with Parkinson’s disease as its most well-known representative. The molecular mechanism behind the aggregation of α-Syn is still not completely understood. Many aggregation-prone proteins, like α-Syn, are known to form phase-separated condensates and recent findings suggest that the early events that lead to protein aggregation, amyloid deposition, and ultimately neurodegeneration, are happening within these condensates. Recent work from Piroska et al. published in the journal Science Advances found strong evidence of a connection between phase separation and aggregation of α-Syn. When α-Syn was present in form of a fusion protein with GFP and fragments of a faulty chaperone that facilitates multivalent interactions and formation of phase separated condensates, treatment of SH-SY5Y cells with pre-formed fibrils (PFFs) leads to formation of solid structures forming needle-like protrusions within a few hours. These α-Syn condensates show the characteristics of amyloid deposits, as they are not dissolved by detergents and stop exchanging components with the surrounding cytoplasm. Moreover, they are intensely labelled by Amytracker 630, confirming the presence of amyloid structures within them, while α-Syn condensates formed before exposure to pre-formed fibrils are not. In other words, when α-Syn separates into condensates it is more prone to aggregation and this is probably due to the elevated local concentration of α-Syn within these aggregates. Linking supersaturation of α-Syn in phase-separated compartments with amyloid formation gives important insights into the early stages of neurodegenerative conditions and might provide clues on how to interfere with preventative measures.
Image: Pre-formed fibrils trigger the evolution of α-Syn condensates into solid-needle-like structures in SH-SY5Y neuronal cells. Nuclei (cyan), pre-formed α-Syn fibrils (red), Amytracker (yellow), and α-Syn-GFP (green). Image from Figure S6B, Piroska et al. (2023) Science Advances, 9(33), eadg5663 (CC-BY-4.0).
Read More:
FL-OPTIR for studying biophysical properties of amyloids in cells and tissues
Infrared (IR) spectroscopy is an invaluable tool to study the biophysical properties of amyloid aggregates. Although historically used as a tool for composition analysis of chemical compounds as it measures the vibrational energy of chemical bonds, IR spectroscopy provides measures to analyse the size and rigidity of amyloid fibrils. Coupling of IR spectroscopy to an optical microscope (OPTIR) is a new method that allows users to obtain chemical information of biological macromolecules from cells and tissues at submicron resolution. Using OPTIR for investigating the biophysical properties of amyloid fibrils in cells and tissues has been challenging since amyloid fibrils cannot be easily identified by conventional light microscopy. Therefore, Oxana Klementieva and her research group at Lund University in Sweden combined epifluorescence imaging and OPTIR in a single instrument. Their “Fluorescence-Located” OPTIR or FL-OPTIR allows precise targeting using epifluorescence signals to locate amyloid-rich regions for OPTIR analysis. In a recent study published in Journal of Medicinal Chemistry, Prater et al. used fixed sections of APP/PS1 transgenic mouse brain and labelled amyloids using Amytracker 520. Guiding OPTIR measurements via the fluorescent signal from Amytracker 520, detailed biophysical analysis of amyloids in the core, corona, and outside of the plaque could be performed indicating a high degree of heterogeneity in the distribution of β-sheet structures within the amyloid plaque. Therefore, the FL-OPTIR method with the optotracer Amytracker might be valuable in studying amyloids in cells or tissue.
Image: FL-OPTIR spectra recorded from the outside, core, and plaque corona as indicated by the markers of the corresponding colour on the inset representing an amyloid plaque stained with Amytracker 520. Image from Figure 2D, Prater et al. (2023) Journal of Medicinal Chemistry 66(4), 2542–2549 (CC BY 4.0).
Read More:
Spatial pattern of microglial activation in relation to amyloid plaques
Microglia cells play a vital role in regulating brain development, maintenance of neuronal networks, and injury repair. Their involvement in the progression of protein aggregation leading to neurodegenerative diseases is complex. While they can clear amyloid plaques and prevent their accumulation, dysfunctional microglia regulation may be a key factor in disease progression. Microglia can “sense” accumulation of amyloid plaques which leads to a change in the expression patterns of Plaque Induced Genes (PIGs). One well-known PIG is Trem2, a microglial membrane receptor protein linked with Alzheimer’s disease (AD). A key function of Trem2 is to induce the microglia cells towards a phagocytic phenotype. Therefore, mutations in Trem2 lead to a higher risk of AD. In a study published by Wood et al. in Cell Reports, researchers set out to understand how Trem2 activation works and how it is related to the distance from a plaque. The researchers used spatial transcriptomics to investigate the spatial distribution of gene expression in NLF mice and NLF mice with R47H mutation in Trem2 which is considered a major risk factor for developing AD. To be able to define regions of interest for transcriptomics, Amytracker 520 was used to label plaques. Based on Amytracker 520 signal, radial regions were defined as “on-plaque”, “periplaque” or “away from plaque”. Based on this categorisation, the researchers were able to determine that, from a total of 55 PIGs, 23 (including Trem2) were only upregulated in “on plaque” regions. The significant expression differences between “on-plaque” and “away from plaque” regions observed in mice with functional Trem2 were not apparent in the mice with mutated Trem2. Looking into the functional aspect, the researchers found that, close to plaques, microglia cells express a higher level of CD68 indicative of a phagocytic character. This phenotype was, again, not apparent in mice with mutated Trem2 indicating that the phagocytic phenotypic change of microglia (i.e. CD68 expression) is dependent on the Trem2 genotype. The study highlights the importance of functional microglial activation via Trem2 and the spatial pattern of Trem2 related microglial activation in relation to plaque formation.
Image: Hippocampal section of PFA fixed NLF mouse brain, labelled with Amytracker 520 (green) for amyloid plaques and CD68 (red) indicating the phagocytic phenotype of microglial cells in close proximity to the amyloid plaque. Image from Figure 5C, Wood et al. 2022 Cell Reports 41, 111686 (CC BY 4.0).
Read More:
Amyloidogenicity of SARS-CoV-2 spike protein
Infection with SARS-CoV-2 leads to patients developing the COVID-19 disease, which is a complex hyperinflammatory syndrome, characterised by acute respiratory distress (ARD). Aside from these severe respiratory symptoms, we now know that the virus can present in unexpected, varied, and long-lasting manners. Recent studies hint towards the amyloidogenicity of the SARS-CoV-2 spike protein (Nyström et al. 2022) and indicate the presence of amyloid aggregates in plasma clots from patients suffering from Long-Covid symptoms (Pretorius et al. 2021)
Researchers from the University of Lund in Sweden and the National University of Singapore previously found that spike protein binds to a range of hydrophobic molecules including bacterial lipopolysaccharide (LPS). This is a highly interesting finding considering that the Covid-19 disease is linked to hyperinflammation and LPS binding to pattern-recognition receptors, which play a key role in innate immunity, kickstarting inflammatory processes. The LPS binding sites on the spike protein were found adjacent to regions that are considered aggregation-prone, including the ones that are able to form amyloid structures, suggesting that LPS might modulate aggregation through these residues. In their most recent publication, Petrlova et al. wondered whether LPS contributes to the amyloidogenic properties of spike protein. Using available structures of dimers and trimers of spike protein, they simulated aggregation in presence of LPS and their results suggested that spike protein and lipid-A (a major component of LPS) can form stable higher-order complexes that the spike protein on its own cannot form. The researchers used Amytracker 680 to visualise aggregates formed by spike protein and found small and rounded aggregates (0.02-0.2 µm). When the spike protein was treated with LPS, these aggregates increased to form particles with up to 2 µm. Although, amyloid formation of spike protein and LPS warrants further investigation to study its relevance in vivo. However, understanding the link between spike protein aggregation and amyloid formation will have important implications for diagnostic and therapeutic approaches
Read More:
Other Resources:
Lewy body formation in seeding based neuronal models
Parkinson’s disease is a brain disorder that causes unintended or uncontrollable movements, such as shaking, stiffness, and difficulty with balance and coordination. Symptoms usually begin gradually and worsen over time. As the disease progresses, people may have difficulty walking and talking. The basis of these symptoms is progressive neurodegeneration and accumulation of degradation-resistant intracellular aggregates, termed Lewy bodies, throughout the brain.
One of the major components of Lewy bodies is α-synuclein, a small protein that normally localizes at the presynaptic terminal of neurons where it regulates the release of neurotransmitters and possibly participates in the assembly of the cytoskeleton. The main characteristic of α-synuclein is that it is not tightly folded like most other proteins, but some regions of it can adapt to the shape of its interaction partners. The intrinsically disordered structure of α-synuclein also makes it prone to aggregation. Aggregated α-synuclein disrupts mitochondrial function and therefore leads to neuronal death. Moreover, aggregates of α-synuclein can act in a prion-like manner by seeding further aggregations, and spreading from one region of the brain to others, partially explaining the progressiveness of Parkinson’s disease.
The molecular mechanism behind α-synuclein aggregation and its correlation with Lewy body formation and neurodegeneration is poorly understood, and appropriate models for studying α-synuclein aggregation are lacking. The most common model for α-synuclein fibrillization is a seeding-based approach in which formation of intracellular aggregates is induced by adding a small amount of α-synuclein fibrils to a neuronal culture. Normally, the cells are then kept in culture for 14 days and α-synuclein fibrillization is studied. Under these conditions, however, only few cells show inclusions that resemble Lewy bodies.
Researchers from the lab of Professor Hilal A. Lashuel at the Ecole Polytechnique Fédérale de Lausanne (EPFL) in Switzerland hypothesized that the maturation process of transitioning from α-synuclein fibrils to Lewy bodies might require a longer time than designated by the standard model. Therefore, the authors prolonged incubation time from 14 to 21 days. At this later time point, inclusions with both an immunocytochemical profile and morphological structure similar to bona fide Lewy bodies were observed in about 20% of the neurons.
To characterize the effects of α-synuclein seeding over time, the authors used an antibody to label phosphorylated α-synuclein (pS129) which is the predominant form in Lewy bodies. In addition, they used antibodies labeling proteins that are a common component in Lewy bodies, like α-synuclein as well as ubiquitin and ubiquitin binding protein (p62) to characterize the effect of α-synuclein seeding. Finally, they used Amytracker 680 to label structures containing repetitive β-sheets to confirm the amyloid-like nature of the seeded aggregates. Indeed, Amytracker 680 colocalized with phosphorylated α-synuclein at days 7, 14 and 21 after seeding. While the seeded aggregates shared the immunohistochemical profile of Lewy bodies already at an early stage, the morphological features of bona fide Lewy bodies like their association with various organelles, including mitochondria became evident only at a later stage. It was observed in a few cases after 14 days, but was much more evident after 21 days.
Due to the observed association of Lewy Body-like inclusions with mitochondria, the authors investigated how the association with Lewy bodies affects mitochondrial function. They found that 21 days after the seeding of α-synuclein, but not after 7 or 14 days, mitochondrial respiration was severely impaired confirming that the formation of Lewy bodies might be associated with mitochondrial dysfunction. Using transcriptomic analysis, the authors showed that the expression of genes that regulate neuronal cell death was significantly altered at the later time points of the study. Furthermore, α-synuclein mRNA levels were significantly reduced at day 21 suggesting that the neurons are trying to prevent any further aggregation.
In summary, the publication by Mahul-Mellier et al. makes a strong point for increasing incubation times in seeding based models to be able to study the processes leading to the maturation and formation of Lewy bodies, which can reveal new insights into the pathogenesis of Parkinson’s disease.
Read More:
Other Resources:
Prolonged stress leads to accumulation of misfolded proteins in the nucleolus
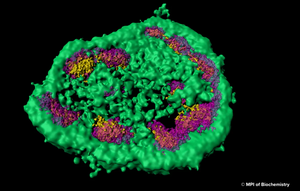
The nuclear proteome is rich in proteins which are prone to aggregate upon conformational stress. This might explain why intranuclear inclusions can often be found in neurodegenerative disorders associated with protein aggregation. Using a combination of fluorescence imaging, biochemical analyses, and proteomics, researchers at Max Planck Institute for Biochemistry around Prof. F.-Ulrich Hartl have investigated the role of the nucleolus as a “phase-separated protein quality control compartment” and published their results in the renowned scientific journal Science. The nucleolus is the largest non–membrane-bound nuclear subcompartment and consists of liquid-like phases that do not intermix, giving rise to distinct zones. To investigate the fate of a nuclear protein during heat stress, the researchers generated a cell line producing a reporter protein called NLS-LG. NLS-LG carries a nuclear localization signal, to make sure it is distributed to the nucleus. The protein's location can be tracked by a heat stable fluorescent signal and its folding state can be tracked with a thermolabile fluorescent signal. When the researchers exposed the cells to heat, they saw that NLS-LG was unfolded and transferred into the outermost zone of the nucleolus. (Figure: The outermost zone of the nucleolus is the granular component (GC) phase marked in green.) Upon recovery from heat stress, NLS-LG was again relocated to the nucleus and a bright fluorescent signal indicated successful refolding. It was further shown that Hsp70, which is an important part of the cell's machinery for protein folding, also transferred to the nucleolus upon heat stress. When the activity of Hsp70 was inhibited, relocation of NLS-LG to the nucleus after recovery from heat stress was also prevented.
z¨Figure: Super-resolution light microscopy shows that the nucleolus is not separated from the rest of the cell by a membrane and that it consists of different zones, which are distinct from each other and membrane-less as well. © MPI of Biochemistry)
When nucleolar organization was disrupted by a toxin that causes nucleolar disassembly and the cells were exposed to heat, instead of translocating to the nucleolus, the NLS-LG reporter protein formed aggregate foci in the nucleus. Using Amytracker 680, the authors were able to demonstrate that these nucleoplasmic foci, in contrast to nucleolar assemblies, were positive for amyloids with a cross β structure. Although recovery from heat stress was slow and inefficient in the nucleoplasmic foci, a certain capability of refolding was evident since Amytracker 680 fluorescence intensity decreased upon recovery from heat stress. This demonstrates that Amytracker 680 can also be used to label reversible aggregates on top of irreversible amyloid fibrils. To explore the protective capacity of nucleolar quality control, cells were exposed to prolonged heat stress. This led to an increase in nucleolar volume attributed to the influx of misfolded protein and to a transition in nucleolar appearance from liquid droplet-like to a hardened state. When Amytracker 680 was used under these conditions in cells expressing the NLS-LG reporter protein, a distinct nucleolar signal indicated presence of amyloids with a cross β structure, which dissolved slowly upon recovery from heat stress. This shows the potential of Amytracker 680 to report the presence of amyloids in phase separated compartments like nucleoli highlighting Amytracker as a tool to be used in in vitro liquid-liquid phase separation experiments for studying protein aggregation and amyloid formation.
Taken together, the publication by Frottin et al. pioneers the notion that “the nucleolus serves as a storage compartment for a subset of misfolded proteins under proteotoxic stress conditions, preserving them in a state competent for refolding or degradation”. The impairment of nucleolar quality control through environmental stressors and other factors might directly contribute to the emergence of idiopathic neurodegenerative pathology.
Read More:
Amytracker can be used for intracerebral multiphoton microscopy
A team of researchers from Massachusetts General Hospital and Harvard Medical School as well as Linköping University have used a fluorescent probe of the same type as Amytracker for multiphoton imaging of Amyloid-β deposits in transgenic mice in vivo.
The fluorescent molecule clearly targeted and labeled core plaques in the cerebral tissue and vasculature when imaged after intravenous injection. The fluorescent signal appeared already shortly after injection, however reaching maximal intensity between 24 and 72 hours – clearly showing the capacity of the probe to pass the blood brain barrier without causing toxicity. Since core plaques are labeled intensely with the fluorescent molecule, emitting in the red channel, the molecule can also be combined with other markers for diffuse plaques or neurofibrillary tangles with emission in the green or blue channel.
Read More:
The clue to detect multiple systems atrophy?
In a study, recently published in Nature, a fluorescent tracer molecule similar to Amytracker has been used to detect α-synuclein aggregates in cerebrospinal fluid from patients with synucleopathy. Interestingly, the fluorescent tracer molecule was binding aggregates from patients with Multiple Systems Atrophy with higher affinity than aggregates from patients with Parkinson’s Disease. While aggregates of α-synuclein in distinct synucleinopathies have been proposed to represent different conformational strains of α-synuclein, this is the first study that provides distinct clues that can be used in future diagnostic assays.
Read More:
Anomalous fibrin amyloid formation
Lipopolysaccharides (LPS) from the Gram-negative cell envelope can be shed from dormant bacteria or from continual bacteria entry into the blood and serve to contribute to the chronic inflammation. The presence of highly substoichiometric amounts of LPS from Gram-negative bacteria caused fibrinogen clotting to lead to the formation of an amyloid form of fibrin. The teams around Prof. Douglas B. Kell from the University of Manchester and Prof. Etheresia Pretorius from the Stellenbosh University have shown that the broadly equivalent lipoteichoic acids (LTAs) from two species of Gram-positive bacteria have similarly (if not more) potent effects than LPS. Specifically they have showed that LPS, iron, and the 2 LTAs cause amyloid formation of plasma proteins, and in particular of fibrin(ogen) as blood is clotted. They confirmed amyloidogenesis by using Amytracker 480 and Amytracker 680 to identify amyloid protein deposits.
Read More:
Amyloids in type-2 diabetes
Type-2 diabetes is a progressive condition marked by resistance towards the blood-sugar regulating hormone insulin. Recently, Type-2 diabetes is become recognized as an inflammatory condition which is often accompanied by cardiovascular complications. The teams around Prof. Etheresia Pretorius from Stellenbosh University and Prof. Douglas B. Kell from the University of Manchester investigated blood clot formation from plasma of diabetic patients. Using advanced confocal microscopy and Structured Illumination Superresolution Microscopy, the authors found that plasma clots from diabetic patients contain significant amounts of amyloid hinting towards an inflammatory cue for the occurrence of cardiovascular symptoms in Type-2 diabetes.
Read More:
Advanced imaging
In a new study in the Journal of visualized experiments, the team around Prof. Peter Nilsson and Prof. Per Hammarström from Linköpings University describe how luminescent conjugated oligothiophenes (LCOs) can be used with Hyperspectral Imaging (HIS) and Fluorescence Lifetime Imaging (FLIM) to detect amyloid species. In a practical approach, the authors highlight caveats of LCO staining and give valuable advice on troubleshooting.
Read More:
Protein engineering for better PET radioligands
An approved method for visualization of Amyloid β (Aβ) plaques in patients suffering from Alzheimer's disease is Positron Emission Tomography (PET). This method requires a radiolabeled amyloid ligand. A frequently used molecule is Pittsburgh Compound B (PIB) which is a derivative of Thioflavin T. Radiolabeled PIB is well-suited to visualize insoluble Aβ plaques and has been used for differential diagnosis of AD. However, the PIB signal, giving an estimate of total plaque load becomes saturated early in the disease progression. To being able to diagnose Alzheimer's disease early-on, soluble forms of aggregated Aβ like oligomers and protofibrils need to be visualized
Generally, monoclonal antibodies have been extremely successful in determining biological target structures and are a well-known diagnostic and therapeutic tool. Yet, transport of antibodies through the blood brain barrier is restricted. Previously, it has been reported that the brain uptake of antibodies can be increased significantly by adding specificity to the Transferrin Receptor (TfR).
In the current study, presented by a team of scientist from Uppsala University a monoclonal antibody against Aβ is coupled to and antibody against the transferrin receptor. When radiolabeled, this construct can be used as a PET probe. In the study, transgenic mice were used as a model for Alzheimer's disease. PET imaging using the new probe was performed in these mice and it was shown that the amount of probe increased with the degree of Aβ pahology to detect soluble forms of Aβ. Amytracker was used in this study for histopathological analysis in tissue sections from transgenic mice.