Amytracker can be used to label protein aggregates in tissue sections or cells prepared by the most common techniques. It can be used in freshly sliced tissue without fixation but also in fixed cells or sections obtained from flash-frozen or paraffin embedded tissues. Generally, fixation in 4% PFA works well for extracellular deposits, and fixation in ice-cold ethanol or acetone is recommended for best preservation of intracellular aggregates. Amytracker can be easily combined with your co-staining of choice. As Amytracker are only fluorescent when bound to their target, washing steps might be omitted and it is possible to add Amytracker to the mounting medium or just before mounting. It is not necessary to protect Amytracker from light, but you should take care not to let Amytracker dry on the sample.
Materials and Solutions:
-
Amytracker - Aqueous or Amytracker - DMSO or Amytracker - Drop&Shine
- Phosphate buffered saline (PBS), pH 7.4
- tissue sections on microscope slide
- ice-cold ethanol, 95%
- Deionized water
- Mounting medium
Assay Procedure:
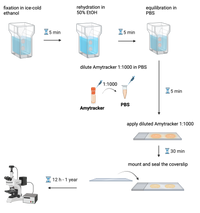
- Fix tissue sections with method of choice. We recommend fixation with ice-cold ethanol (5 min) at room temperature.
- Place microscope slide with tissue sections in a staining jar filled with ice-cold ethanol, 95% for 5 min.
- Rehydrate tissue sections in a mix of ethanol and deionized water (1:1) for 5 min. The rehydration step may need to be repeated with lower ethanol ratio depending on the tissue.
- Equilibrate sections in PBS for 5 min.
- If you are co-staining Amytracker with antibodies, perform blocking steps according to your experimental needs, but avoid incubating Amytracker in the blocking solution. Therefore, Amytracker should be used after antibody staining, just prior to- or during mounting.
- Dilute Amytracker in PBS 1:1000.
- Apply diluted Amytracker generously to the tissue section. Use enough liquid to prevent the sections from drying out during incubation. Incubate for 10-30 min.
- For an easy solution to add Amytracker while mounting, use our Amytracker - Drop&Shine instead of another mounting medium.
- Mount tissue sections and seal the coverslip onto the slide to prevent drying.
Imaging:
- Perform fluorescence imaging on the following day or after. Store slides in dark at RT. Amytracker fluorescence should be detectable in labeled, properly stored and handled sections for at least one year.
Read more →
Amytracker can be used for intravenous- or intraperitoneal injection in small animals to label protein aggregates in vivo. It will readily cross the blood brain barrier and can be imaged by intra-vital microscopy or after removing the tissue and preparation of microscope slides. For systemic injection, we recommend to use our Amytracker - Solid formulation that comes in sterile injection bottles.
Solutions and Reagents:
- Amytracker - Solid 1mg
- Physiological saline
- Injection syringes and needles
Assay Procedure:
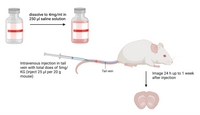
- Add 250 µl physiological saline to the Amytracker - 1mg Solid by injecting it directly through the rubber stopper. Dissolve all powder residues by shaking or vortexing the bottle.
- Inject a total dose of 5mg/KG of Amytracker intravenously or intraperitoneally.
- You can expect to see staining of cerebral plaques 24-h up to 1 week after injection.
Readout
- For multiphoton microscopty, all Amytracker variants can be excited with a tuneable laser at 800 nm. See the table below for excitation and emission properties of all Amytracker variants with standard microscopy methods.
Read more →
This protocol describes how Amytracker can be utilized for fibrillation assays and detection of amyloids in liquid samples. As all Amytracker variants are highly fluorescent only when they are bound to their target, they are ideally suited for spectrophotometric analysis. We recommend to perform a titration to use Amytracker in the lowest concentration possible for your specific application. The experimental conditions used to induce protein misfolding and aggregation can vary considerably depending on the amyloidogenic protein or peptide. It is important to note that Amytracker fluorescence can vary depending on pH and ionic strength of the buffer. In this protocol, fibrillation of bovine insulin is performed in 2 M acetic acid and 0.5 M NaCl.
Materials and Reagents:
- Amytracker - DMSO
- Fibrillation solution: 2 M acetic acid and 0.5 M NaCl in deionized water
- Bovine insulin
- Deionized water
- 96-well microtiter plate with lid.
- Plate reader
Assay Procedure:
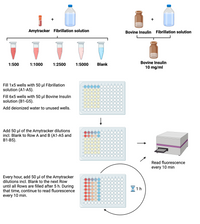
- Prepare a dilution series of Amytracker (1:500, 1:1000, 1:2500, 1:5000) in Fibrillation solution and include a blank control (Fibrillation solution without Amytracker).
- Prepare 10 mg/ml bovine insulin in Fibrillation solution.
- Fill 1x5wells with 50 µl Fibrillation solution.
- Fill 6x5wells with 50 µl Bovin Insuline solution.
- Fill the rest of the wells with deionized water to avoid evaporation.
- Add 50 µl of each Amytracker dilution incl. Blank to Row 1 & 2.
- Place the lid on the microtiter plate
and read emission every 10 min for 5 hours in total.
- Every hour, remove the plate from the Reader and add Amytracker dilutions incl. Blank to the next row filled with Bovine Insulin solution.
Readout
- Make sure to read from the bottom of the plate. Note that excitation and emission are different for each Amytracker variant and excitation- and emission maximum can slightly vary for each target. For reference excitation and emission settings, see table below.
Read more →
All Amytracker variants cross the cell membrane of living cells without permeabilization. Due to their low background fluorescence and minimal interference with biological autofluorescence, we recommend Amytracker 630 or Amytracker 680 for live-cell imaging. As Amytracker do not bleach easily, they are excellently suited for repeated illumination during time-lapse imaging. If possible, use serum-free medium during incubation.
Solutions and Reagents:
-
Amytracker - Aqueous or Amytracker - DMSO
- Imaging medium: Serum- and Phenol Red free cell culture medium
Assay Procedure:
- Dilute Amytracker in Imaging medium 1:500.
- Incubate your cells in Imaging medium for 30 min under normal culture conditions.
- Image cells
- Replace Imaging medium with standard cell culture medium.
Readout:
Perform imaging using the filter settings listed in the table below.
Read more →
