Amyloidosis is the name for a group of conditions caused by a build-up of amyloid protein deposits in organs and tissues throughout the body. A great many diseases can be classified as amyloidosis. The most well known are the cerebral amyloidoses Alzheimer's and Parkinson's disease. Lesser known are the systemic amyloidoses and Type-2-Diabetes. Although some genetic determinants have been identified, the large majority of patients suffering from amyloidosis have no identified family history, meaning the disease is sporadic and acquired. As symptoms in the early stages of the disease are often diffuse and mimic those of other conditions, diagnosis is difficult. Therefore, identification of risk factors is an important tool that can be used to facilitate early diagnosis. While it is well known that patients with a chronic infectious or inflammatory disease may be at greater risk of developing secondary amyloidosis, the link between inflammation and protein aggregation hasn’t been well described.
A series of publications from the research group around Prof. Resia Pretorius at Stellenbosch University in South Africa describes the relationship between inflammation and abnormal coagulation. Their studies propose that abnormal coagulation might be the missing link between inflammation and protein aggregation. Prof. Pretorius’ early work has been based on the finding that many supposedly non-communicable diseases like stomach ulcers actually have a bacterial or viral origin. A bacterial link has also been proposed in the aetiology of Alzheimer’s disease, Parkinson’s disease and even implicated in Type-2-Diabetes. Earlier work of Prof. Pretorius together with Prof. Douglas B Kell at University of Manchester in the United Kingdom describes how dormant bacteria, unrecognized by microbiological testing, can circulate in the blood and exit from dormancy through a dysregulation of iron metabolism and/or stress. The reactivation from dormancy can release bacterial inflammagens that lead to an activation of the immune system and cause an inflammatory response, which is linked to abnormal clotting or hypercoagulation caused by amyloidogenic fibrin(ogen).
In the first paper out of a series of three (de Waal et al. 2018), the authors identified a well known bacterial inflammagen - lipopolysaccharide (LPS) - associated with fibrin fibres in blood clots of patients suffering from Parkinson’s disease, Alzheimer’s disease and Type-2-Diabetes. The authors obtained whole blood samples from patients and healthy controls and prepared platelet poor plasma (PPP) which they used to induce formation of PPP clots by adding thrombin. They used Amytracker 480, Amytracker 680 and Thioflavin T to detect amyloids in PPP clots from healthy individuals or individuals with Parkinson’s disease and found that there is more amyloid when clots are formed with fibrin(ogen) of diseased individuals than with fibrin(ogen) of healthy individuals. On top of that, they were able to detect elevated amounts of the bacterial inflammagen LPS in PPP or whole blood smears from diseased patients. Using an advanced imaging technique called correlative light electron microscopy, which allows the overlay of a confocal or super-resolution (fluorescence) micrograph onto a scanning electron micrograph, the authors showed that the fluorescent signal obtained from an anti-LPS antibody is merged and fused into the dense matter of the fibrin(ogen) deposits.
The second publication in the series (Adams et al. 2019) builds on these findings and investigates the link between Parkinson’s disease and a bacterial inflammagen - gingipain - a protease produced by Porphyomonas gingivalis (P. gingivalis), which is common in the oral cavity with the capacity to cause chronic periodontitis. Gingipains, like other bacterial inflammagens, are present on the bacterial surface, but can also be secreted from the bacterium and can enter the circulation. The results of the study showed that whole blood from Parkinson’s disease patients contains elevated levels of pro-inflammatory biomarkers and cytokines. This goes along with platelets showing substantial (hyper)activation, spreading and aggregation together with a tendency for hypercoagulation in whole blood samples. When green fluorescent fibrinogen was incubated with P. gingivalis LPS and treated with thrombin to form clots, fibrin networks display a denser and more matted network. The protease gingipain alone greatly inhibited fibrin network formation, but the effect was limited when incubated together with P. gingivalis LPS. A fluorescent antibody was used to detect gingipain in blood samples and it was evident that gingipain was present in clots obtained from PPP of diseased patients. As also shown in the previous publication, clots obtained from PPP of diseased patients show enhanced fluorescence when labeled with Amytracker 480, Amytracker 680 or Thioflavin T. This result suggests that in clots derived from the blood of Parkinson’s disease patients, fibrinogen polymerizes into a form with greatly increased number of β sheets, resulting in the formation of amyloid fibrils.
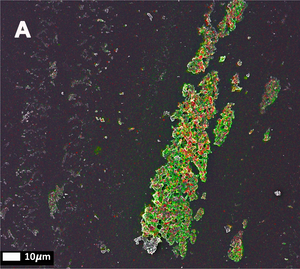
The third publication in the series (Page, M et al. 2019) takes a step away from bacterial inflammagnes and investigates the role of inflammation on anomalous blood clotting. As a marker for inflammation, the authors use the acute-phase protein Serum Amyloid A (SAA). In response to inflammatory processes, cytokines induce SAA production in the liver resulting in up to 1000-fold increase in SAA plasma concentration. At the site of infection, SAA activates the inflammation cascade leading to activation of the innate immune response. In the study, the authors added purified SAA to green fluorescent labeled fibrinogen as well as whole blood or platelet poor plasma (PPP) from healthy donors. The results confirm the observations made using bacterial inflammagens. SAA promoted atypical coagulation and platelet activation. When SAA was added to fibrinogen and clotted with thrombin, amyloid structures were identified after labeling with Amytracker 680, Amytracker 480 or Thioflavin T. When SAA was added to green fluorescent labeled fibrinogen and clotted with thrombin, large areas of hypercoagulable fibrin(ogen) were found. Amytracker 680 was shown to bind in the vicinity of these hypercoagulable areas, pointing towards the fact that anomalous hypercoagulated areas that form in the presence of SSA have an amyloid nature. When SAA was added to PPP of healthy donors, and clots were obtained by adding thrombin, fluorescence of the amyloid markers Amytracker 680, Amytracker 480 and Thioflavin T increased and showed large patches of visible amyloid. Correlative light electron microscopy was used to show close association of SAA, labeled using a green fluorescent antibody, and amyloid structures, labeled by Amytracker 680 (Figure from Page M, et al. 2019: Correlative Light Electron Microscopy confirms the presence of SSA in amyloidogenic fibrinogen clots, CC BY 4.0).
Taken together, the publications present evidence that bacterial inflammagens or innate inflammatory proteins in the circulation lead to anomalous clotting and the formation of amyloid structures in fibrin(ogen) clots. These amyloid structures seem to be associated with the causative inflammagens. Amyloid structures in plasma clots from patients with cerebral or systemic amyloidosis closely resemble those caused by various inflammagens and specific bacterial inflammagens can be detected in anomalous clots from diseased patients. These results strongly point towards the fact amyloid aggregates in abnormal blood clots might present a risk factor for amyloidosis.
Read More:
- Kell, DB & Pretorius, E (2018) “No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases” Biological Reviews 93(3), 1518-1557
- Kell, DB & Pretorius, E (2018) “No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases” Biological Reviews 93(3), 1518-1557
- Pretorius, E et al. (2017) “Substantial Fibrin Amyloidogenesis in Type 2 Diabetes Assessed Using Amyloid-Selective Fluorescent Stains.” Cardiovascular Diabetology 16(1), 1–14
- de Waal, GM et al. (2018) “Correlative Light-Electron Microscopy Detects Lipopolysaccharide and Its Association with Fibrin Fibres in Parkinson’s Disease, Alzheimer’s Disease and Type 2 Diabetes Mellitus.” Scientific Reports 8(1), 1–12
- Adams, BJ et al. (2019) “Parkinson’s Disease: A Systemic Inflammatory Disease Accompanied by Bacterial Inflammagens.” Frontiers in Aging Neuroscience 10, 1–17
- Page, MJ et al. (2019) “Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation.” Scientific Reports 9(1), 1–14
