Under some conditions, peptides or proteins may convert from their soluble forms into unsoluble highly ordered fibrillar aggregates. These aggregates may cause disease through various mechanisms. Prominent examples of aggregating peptides related to neurodegenerative diseases are Amyloid β peptides which play an important role in Alzheimer's disease and aggregating α-synuclein which is found in Lewy bodies that are a hallmark of Parkinson's disease and some forms of dementia. Multiple variants of systemic amyloidoses are caused by aggregating immunoglobin light chains, fragments of serum amyloid A protein or aggregating transthyretin. But many more forms of systemic and localized amyloid diseases exist.
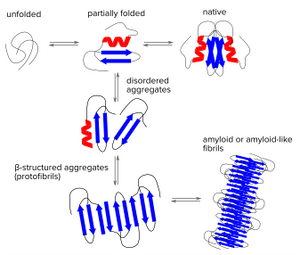
Protein folding is a delicate process in which partially folded polypeptide structures are vulnerable to forming disordered aggregates. These disordered structures might dissociate again, but under certain conditions they will get stuck and form very stable β structured aggregates (protofibrils) which can grow into mature fibrils by further self-association or repetitive addition of monomers. Thus, regardless of the aggregating polypeptide chains share any sequence homology, the β-sheet is the structural hallmark of many types of fibrillar aggregates called Amyloids. Heterogenic features of amyloids might be due to the alignment of adjacent strands and the separation of the amyloid structures.
